| Citation: | Please cite this article as: Muntané-Carol G, Romaguera R, Gómez-Hospital JA, Nuche J, Philippon F, Rodés-Cabau J. Management of conduction disturbances after TAVI: the last step towards early discharge. J Geriatr Cardiol 2025; 22(5): 534−546. DOI: 10.26599/1671-5411.2025.05.004. |
The incidence of new-onset cardiac conduction disturbances following transcatheter aortic valve implantation (TAVI) has not decreased compared to other complications, and nowadays is by far the most frequent drawback following the procedure. Meanwhile, the global management of TAVI recipients has led to a minimalist approach with short postprocedural length of stay, which may be limited by the occurrence of late arrhythmic events in patients at high-risk. This review focuses on those strategies to overcome the conundrum between early discharge and new-onset conduction disturbances in elderly TAVI candidates and provides a perspective on future improvements in this field.
Degenerative aortic stenosis (AS) is the most common primary valve disease leading to intervention in Europe and North America, and its burden will increase further as the population ages.[1,2] The development of transcatheter aortic valve implantation (TAVI) for the treatment of AS may be considered the most significant paradigm shift in the field of cardiology in recent decades. Since the first case performed more than 20 years ago, solid scientific evidence set the indication for most patients with severe AS.[3,4] Thus, the most recent European clinical guidelines recommended transfemoral TAVI as the preferred treatment for elderly patients (≥ 75 years old) with AS.[4]
In recent years, advancements in TAVI implantation techniques, increased operator experience, and enhanced transcatheter heart valve (THV) designs have resulted in reduced perioperative mortality and lower procedural complication rates.[5,6]
However, and unlike other periprocedural complications, the incidence of new-onset cardiac conduction disturbances (CDs) has not decreased significantly over time, and nowadays is by far the most frequent drawback following TAVI. New-onset persistent left bundle branch block (LBBB) and permanent pacemaker implantation (PPI) occur at rates of 14.4% and 9%-16% respectively, according to recent large-scale data from the STS-ACC transcatheter valve therapy registry.[7,8] In addition, the management of TAVI recipients has evolved significantly since the beginning of the technique, leading to a minimalist approach with a short (24–48 h) postprocedural length of stay (LOS).[9–11] However, this strategy could be limited by the occurrence of late (> 48 h) life-threatening arrhythmic events, particularly in patients at high risk for complete heart block after TAVI such as those with prior right bundle branch block (RBBB) or new-onset CDs.[12–14] Initial evidence showed that while LOS has declined in recent years, the rate of readmission for PPI has increased significantly.[15,16]
This review focuses on those strategies to overcome the conundrum between early discharge and new-onset CDs in elderly TAVI candidates and provides a perspective on future improvements in this field.
The close proximity of the conduction system, particularly the bundle of His and the left bundle branch, to the base of the non-coronary and right-coronary leaflets is the main reason for CDs after TAVI.[17] Interaction with the cardiac conduction system during TAVI occur during wire insertion, valve implantation, and pre/post balloon dilatation. The deployed valves can directly damage the conduction system, leading to edema, hematoma, and ischemia,[18] causing transient or permanent worsening of the atrioventricular conduction. Electrophysiological (EP) studies have shown damage to the atrioventricular node, His, and infra-His conduction system during TAVI procedures.[19] Overall, the effects of the THV on the cardiac conduction system may result in new-onset LBBB and high degree or complete atrioventricular heart block (HAVB/CHB), requiring PPI.
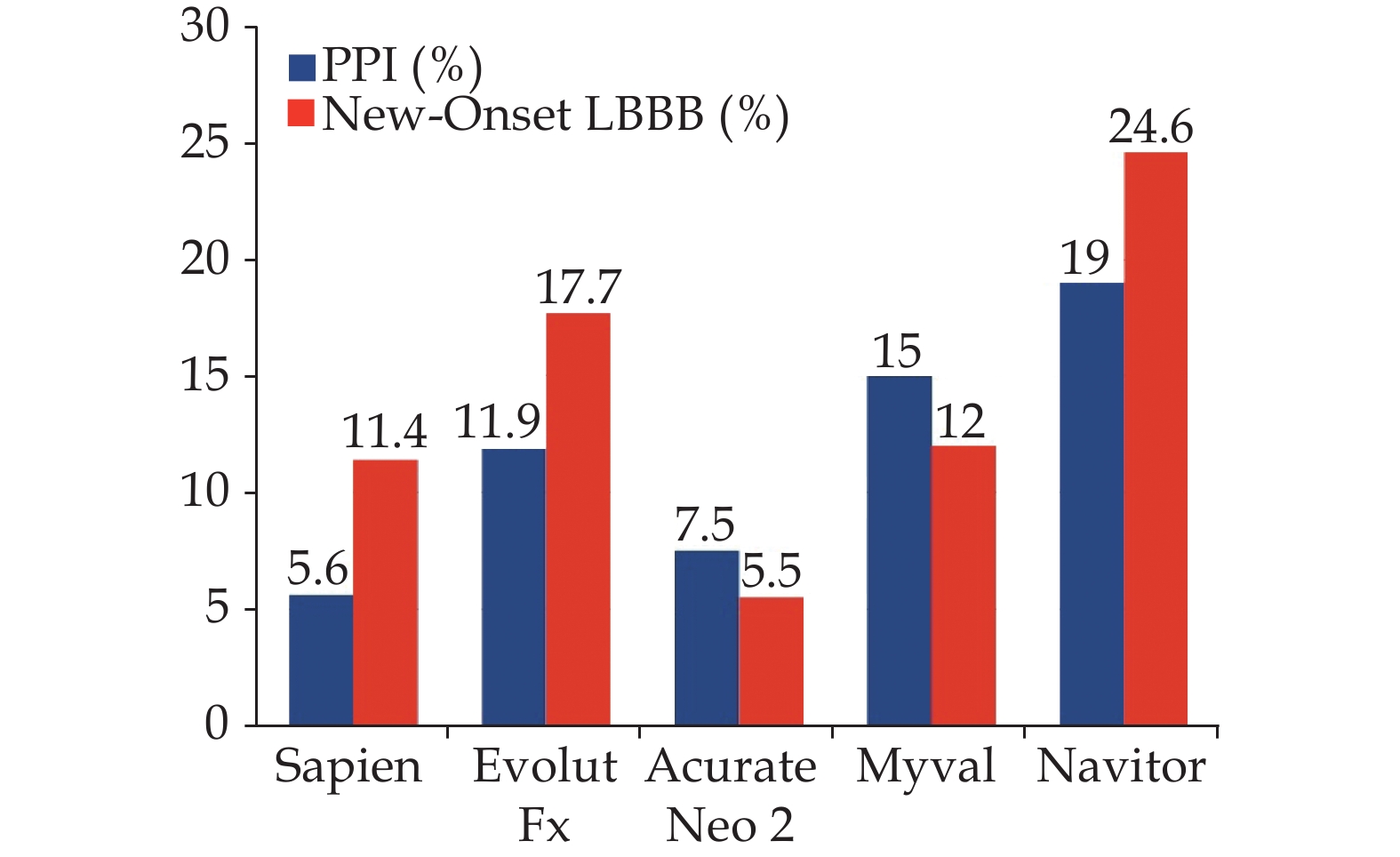
The occurrence of new-onset LBBB remains the most common complication after TAVI. The current new-onset persistent LBBB rate in the TAVI field is 14.4%.[8] However, the incidence of new-onset LBBB among TAVI recipients has been variable due to factors such as the use of different THVs, inclusion of transient LBBB, differences in baseline risk for conduction disturbances, and variations in the timing of electrocardiogram (ECG) acquisition, leading to various definitions of new-onset LBBB. The main predictors for new-onset LBBB included the use of some self-expandable valves, depth of implantation, overexpansion of native annulus and larger valve sizes.[20] Figure 1 illustrates the reported incidence of new-onset LBBB using newer generation THV systems, with a range from 5.5% to 24.6 %.[21–25]
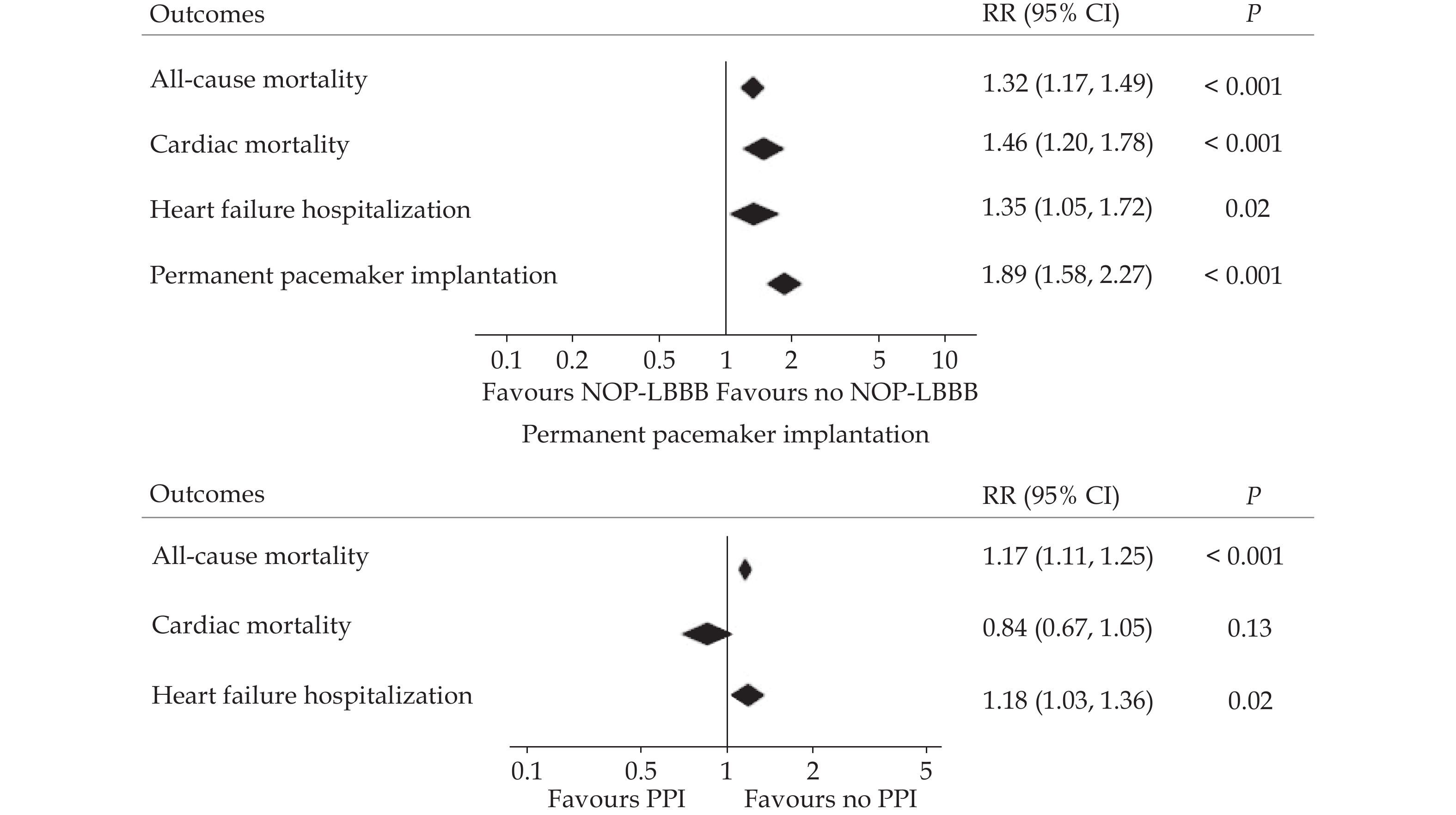
The clinical impact of new-onset LBBB following TAVI may be influenced by the potential progression to HAVB and the negative effect on left ventricular ejection fraction (LVEF). Previous meta-analyses have reported an approximately 2-fold increased risk of PPI after TAVI.[26,27] However, current data do not support routine prophylactic PPI implantation in these patients. Nevertheless, some studies suggest that patients with a very long PR interval (> 240 ms) and/or a QRS interval duration >150–160 ms may have an increased risk of delayed HAVB and sudden death.[13,14,28,29] On the other hand, although initial reports on mortality and heart failure rehospitalization outcomes in these patients have shown inconsistent results,[30–36] a subsequent meta-analysis showed that new-onset LBBB was associated with an increased risk of all-cause death, cardiac mortality, and heart failure hospitalization (Figure 2).[37]
Regarding PPI, newly designed THVs with improved features such as repositioning and enhanced leak prevention have not reduced PPI rates significantly. The main predictors for PPI following TAVI included baseline RBBB, self-expandable valves, depth of implantation, overexpansion of native annulus, and first-degree atrioventricular block.[20] Figure 1 shows the PPI rate among newer generation devices, which ranges from 5.6 to 19%.[22,24,25,38,39] Although previous data showed higher rates of PPI in self-expandable valves compared to balloon-expandable valves,[20] the available randomized comparisons between these two types of THV systems did not show statistically significant differences.[40,41]
Long-term right ventricular pacing has a deleterious effect on left ventricular function, as demonstrated in other cardiovascular settings.[42–45] Accordingly, a meta-analysis including > 40000 TAVI patients showed an increased risk of all-cause mortality and heart failure hospitalization (Figure 2).[37]
It is important to note that around 90%-95% of new-onset events leading to PPI occur in the acute period,[20] which includes intraprocedural events and those within 48 hours following TAVI. Different studies have used varying definitions for delayed (late) events, but generally, they are established as events occurring more than 48 hours after the procedure.[16] A recent comprehensive review of 19 studies involving 14,898 patients revealed that the occurrence of delayed HAVB following TAVI ranged from 1.7% to 14.6%.[46] Upon further analysis of 5 selected studies with similar definitions for delayed events (≥ 48 h), the estimated incidence was determined to be 5.2%.[46] However, it is essential to note that more comprehensive prospective data is needed to accurately estimate the true incidence of this phenomenon.
In conclusion, the most common complication of the TAVI procedure is the development of new-onset CDs, which are associated with poorer clinical outcomes.
The initial TAVI care pathway was based on surgical programs, including general anesthesia, intraprocedural invasive monitoring, transesophageal echocardiography, surgical main access, and prolonged intensive care unit admission. However, with increased operator experience and technique improvement, there has been a significant reduction in complications and therefore LOS. The TAVI procedure has now evolved into a simplified and “minimalistic” intervention, involving local anesthesia, full percutaneous femoral approach with 14–16 Fr sheaths, radial secondary access, left ventricle wire pacing, and early mobilization. This evolution has resulted in a decrease in LOS, with most TAVI recipients being discharged within 24–48 h, and even same-day discharge in selected cases.[9–11]
Multiple studies have explored the possibility and factors linked to early and safe discharge following TAVI. This encompassed specific patient traits, minimalist peri-procedure approaches, and the absence of post-procedural complications (mainly new-onset CDs), along with early mobilization.[47–53]
The first prospective experience was the multicenter Vancouver 3M (multidisciplinary, multimodality, but minimalist) TAVI study. It showed the feasibility of a clinical pathway with standardized post-procedure care, including early mobilization and next-day discharge.[54,55] The study findings confirmed the safety and efficacy outcomes and reduced 30-day economic costs. Additionally, the multicenter European Feasibility and Safety of Early Discharge After Transfemoral TAVI (FAST-TAVI) registry demonstrated that pre-specified risk criteria (in summary, clinical stability and no procedural-derived complications) can effectively identify patients suitable for safe early discharge (median of 2 days following the procedure).[56,57] More recently, Durand, et al.[58] reported the results of the randomized FAST-TAVI II trial, which compared the use of a dedicated training program to implement 10 quality of care measures to reduce LOS (n = 969 patients) with the standard of care (n = 860 patients). Early discharge was achieved in 58.2% of the intervention group, leading to a significantly reduced LOS compared to the control group [3 (IQR: 3) vs. 4 days (IQR: 3), P < 0.0001].[58] Notably, new-onset CDs were the main reason for failed early discharge in both groups, occurring in 47% of patients with extended hospitalization in the overall population. This underlines that the management of CDs following TAVI remains the main limitation for early discharge in the TAVI setting.
Additionally, there has been a lack of consensus regarding the management of CDs after TAVI since the beginning of the technique.[59] In an effort to address this issue, a scientific expert panel aimed to standardize the post-procedural management of CDs after TAVI and proposed an algorithm based on the prior and post-procedural ECG.[60] However, this tailored pre-specified strategy has not been validated yet.
First, it should be noted that patients who undergo a TAVI procedure and do not experience significant ECG changes or rhythm disturbances during the periprocedural period may be discharged home 24 h following the procedure without further measures or monitoring (48 h may be reasonable in selected cases with baseline ECG-CDs). Previous studies with early and long-term follow-up showed that TAVI patients with no post-procedure ECG changes have a low risk of CDs, making shorter hospital stays feasible.[14,29,61] Furthermore, a prior work using ambulatory ECG monitoring (n = 459) in the setting of minimalist TAVI (median LOS of 2 days) demonstrated that HAVB/CHB events at 30 days were rare in patients without ECG changes post-TAVI (2.2%), and even lower in cases with normal ECG following the procedure (1.2%).[62]
Intraprocedural measures can be implemented to minimize the risk of new-onset CDs. Implantation depth and therefore the interaction with the membranous septum is the main predictor for new-onset CDs.[20] Jilaihawi, et al.[63] reported the results of the MInimizing Depth According to the membranous Septum (MIDAS) strategy. This work demonstrated that a systematic pre-procedural evaluation of membranous septum length along with an adjusted implantation depth in each patient anatomy may reduce the rate of new-onset CDs.[63] In a more simplified way, several studies recommended alternative implantation techniques to achieve a higher (more aortic) valve implantation without an increased risk of complications (e.g., valve migration, significant paravalvular leak, coronary obstruction). In the self-expandable Evolut family, the use of the cusp-overlap projection (isolating the non-coronary cusp to guide the TAVI procedure) provided a better appreciation of the implantation depth. A recent metanalysis pooling data from 11 retrospective studies using the cusp overlap technique group demonstrated lower PPI rates (OR = 0.48; 95% CI: 0.33–0.70; P = 0.001;) and implantation depths (mean difference −0.83; 95% CI: −1.2 to −0.45; P < 0.001).[64] Surprisingly, no differences were found related to new-onset LBBB.[64] On the other hand, the non-repositionable nature of balloon-expandable valves has limited the widespread implementation of techniques aiming at extremely high implantation depths. However, some reports provided promising results with the cusp-overlap technique and the use of the radiolucent line of the unexpanded valve (instead of the balloon marker) to guide the procedure.[65–67]
Baseline RBBB is present in about 10% of TAVI candidates and has consistently been the most significant baseline risk factor for PPI following the procedure, with rates as high as 40%–50%.[20] Also, RBBB is a key indicator of pacemaker dependency during follow up.[68] Additionally, some evidence has shown an increased risk for mortality after hospital discharge in patients with RBBB.[20] In terms of timing of CDs in RBBB patients, a previous study showed that 86% of episodes leading to PPI occurred during the procedure, and 98% within 3 days after TAVI.[69] Furthermore, data from ambulatory ECG monitoring in 38 patients with RBBB (median LOS of 2 [1–4] days following TAVI) confirmed the high-profile risk of patients with RBBB. Severe arrhythmic episodes (CHB/HAVB) occurred after discharge in 13.2% of the patients, highlighting the urgent need to improve post-procedural management in this population.[62]
The high risk of advanced conduction disturbances during the periprocedural period in RBBB patients may hinder early mobilization, which is crucial in some TAVI subgroups (e.g., frail, elderly TAVI candidates). To address this problem, some authors have used temporary active fixation leads connected to an external generator to facilitate mobilization without a significant impact on safety.[70–72] This approach also enables the patient to be transferred to the general ward without requiring a critical care bed.
It is worth considering whether some TAVI candidates with baseline RBBB could benefit from prophylactic PPI implantation. It's important to note that the presence of degenerative aortic stenosis itself is associated with a deleterious effect on the cardiac conduction system, which can lead to conduction abnormalities.[73,74] Accordingly, in three studies involving a total of 582 patients, ambulatory ECG monitoring (ranging from 24 hours to 14 days) was conducted before TAVI.[75–77] HAVB or CHB episodes occurred in 3% of the patients, and a higher rate of bradyarrhythmic events was observed in patients with RBBB.[77] Other studies have examined baseline predictors of PPI in this group of patients.[69,78–80] Advanced age, prolonged PR interval, female sex, degree of calcification at the non-coronary cusp, and membranous septum length have been found as independent predictors of PPI in different studies.[69,78–80] Furthermore, small studies assessed the utility of prophylactic PPI in non-randomized, single-center observational studies.[80–83] A prophylactic pacing strategy was safe, reduced hospital LOS and was cost-effective.[80–83] However, the early and late risks of prophylactic PPI should also be considered. Additionally, a retrospective study using the Acurate Neo valve demonstrated a significantly lower rate of PPI using this THV.[84] However, this finding should be confirmed prospectively.
In summary, prophylactic PPM implantation in very selected and high-risk cases, prolonged post-procedural length of stay of up to 4 days (challenging the “minimalist” TAVI concept in this subset of patients), and close monitoring (ambulatory ECG monitoring using real-time alarm systems that may facilitate the implementation of rapid therapeutic measures) are options to be considered for this particular patient subset.
The management of new-onset LBBB patients remains as an unmet need in the TAVI field.
Various strategies have been implemented in recent years,[59] including clinical observation, prophylactic PPI,[85] AECG monitoring after discharge,[60] or PPI based on EP study result.[86,87] Previous data showed in-hospital PPI rates in this group ranging from 7.6% to 40.9%, but these data should be interpreted with caution due to the use of different indications and definitions.
The MARE study used an implantable cardiac monitor in 103 TAVI recipients with new-onset LBBB and provided data of high clinical relevance.[88] This study showed an incidence of 10% of HAVB/CHB episodes leading to PPI at 1-year follow-up, about half of them during the first month after hospital discharge. In accordance with other studies,[30,89] the MARE study reported a partial or complete recovery of the ECG abnormalities in one-third of patients at 1-year.[88] This underscores the clinical variability that occurs in this subset of patients (from HAVB/CHB requiring PPI to ECG normalization), which makes its management even more challenging.
Faroux, et al.[89] aimed to identify the predictors of LBBB recovery or progression to HAVB/CHB. No clinical, procedural, or ECG variables were identified as predictors of LBBB recovery at follow-up. On the other hand, this work confirmed that a high proportion of patients (nine out of ten) with LBBB will not suffer significant bradyarrhythmias leading to PPI during the first year after TAVI, which strongly discourages prophylactic PPI in such cases. However, those with longer PR intervals or atrial fibrillation had an increased risk of PPI at follow-up.[89] In fact, previous data showed poorer outcomes (increased risk of HAVB and sudden death) in patients with new-onset LBBB and very long PR interval (> 240 ms) and/or wider QRS (> 150–160 ms).[14,28,29] In these patients, a prophylactic PPI may be considered.
As an alternative, the latest European Guidelines propose the use of an EP study to guide the decision for PPI, being recommended if the HV interval is more than 70 ms.[86] In this regard, the recently published LBBB-TAVI study added new data regarding the utility of the EP study in this context.[87] Masssouillé, et al included 183 TAVI recipients with new-onset LBBB.[87] A PPI that recorded AV conduction disturbance episodes was implanted in those patients with His-ventricle (HV) interval > 70 ms (n = 47), and AECG monitoring for 12 months was used in those with HV < 70 ms (n = 136). Patients with HV > 70 ms displayed more high-grade AV conduction disorders (53.2% [25 of 47] vs. 22.8% [31 of 136]; P = 0.001). These results highlighted the need for better stratification of those patients with new-onset LBBB, as half of the group with PPI did not suffer HAVB/CHB episodes.
In conclusion, individualized decision-making will be necessary for patients with new-onset LBBB. For those with stable ECG, normal PR interval, and QRS < 150 ms, discharge 48 h after TAVI may be reasonable. For other cases, a more prolonged hospital stay (3–4 days) following TAVI may be considered. Factors such as intra-procedural details (e.g. pre and post-dilatation, THV type, prosthesis grade of oversizing, implantation depth), day-by-day ECG evolution, and post-procedural clinical tools (EP study, ambulatory ECG monitoring) should guide the clinical management of these patients.
Patients with preexisting ECG conduction disorders exhibiting significant ECG changes (an increase in PR and/or QRS duration of ≥ 20 ms) after TAVI represent a diverse group that requires careful clinical consideration.[60] A similar approach to new-onset LBBB patients (prolonged hospitalization and individualization) may be indicated if PR and/or QRS are > 240 or > 150 ms, respectively, and > 20 ms longer than baseline, particularly if progressive ECG-CDs.
Patients with intraprocedural HAVB/CHB events that persists the day after the TAVI procedure (24h) may receive PPI, as stated in the consensus mentioned earlier.[60] However, current European Guidelines extend the watchful period until 48 h.[86] This is due to the lack of strong evidence in this area, primarily because of the absence of a consistent definition of persistent intraprocedural HAVB/CHB and the paucity of data addressing this issue.
A retrospective study that focused on this matter included 2240 consecutive TAVI patients from two centers.[90] Persistent HAVB/CHB was defined as any procedural HAVB/CHB present at the end of the procedure. Persistent HAVB/CHB occurred in 7.9% of patients (40% with baseline RBBB), leading to PPI in 94% of them. At 30 days, ventricular pacing percentage (VPP) was 97% (ranging from 60% to 99.7%). Global conduction recovery at 30 days (patients with no PPI implantation during hospitalization and VPP < 1% in those with it) was observed in around 10% of the population. Moreover, about 20% had a VPP between 1% and 40% at 30 days. In these patients, one may wonder if conduction recovery during the first days after TAVI may have occurred. However, it is also plausible that these patients experienced intermittent HAVB/CHB episodes, and the pacemaker would have prevented severe clinical events or syncope in these cases. More studies with consistent programming protocols are necessary to determine the true incidence of conduction recovery in this context. In the meantime, it seems reasonable to consider PPI if HAVB/CHB persists 24 h following TAVI. On the other hand, managing patients with transient HAVB/CHB should be individualized, considering the duration of HAVB/CHB and the findings on subsequent ECGs (postprocedural and day 1 following TAVI).
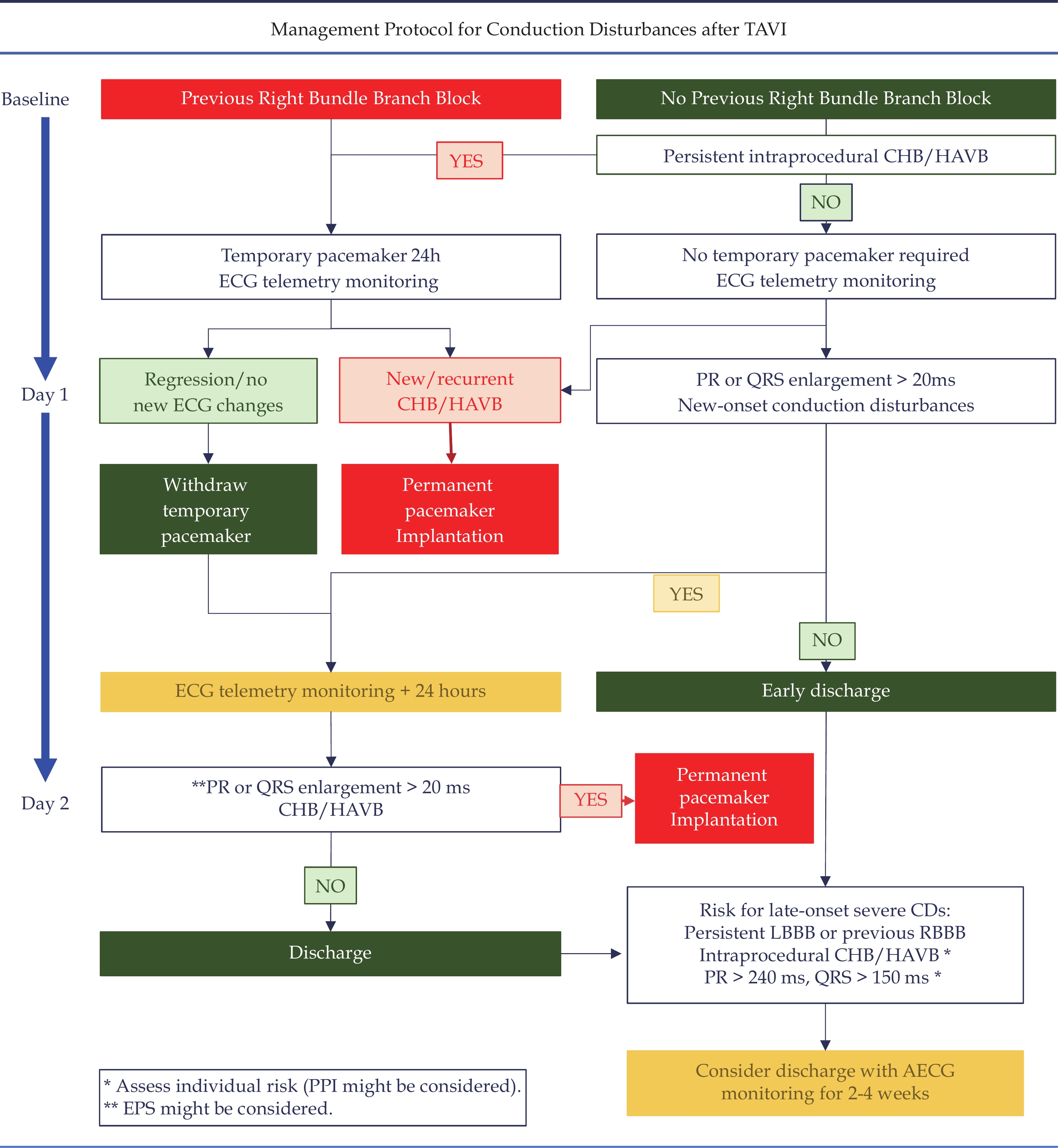
A global algorithm according to the presence of intraprocedural heart block and ECG-CDs following TAVI has been recently proposed (Figure 3), and represents an update from the previously cited consensus.[60,91] A proposed postprocedural LOS assuming the absence of non-arrhythmic complications is shown. In our opinion, discharge 24 h following the procedure may be reasonable in most patients without baseline RBBB or new-onset ECG-CDs. Prolonged hospitalization may be needed according to the risk of HAVB/CHB (Figure 3).
The management of CDs after TAVI will continue to evolve in the coming years to improve TAVI outcomes. Future data may evaluate prophylactic PPI in specific subsets of patients, such as those with baseline RBBB and additional risk factors, in order to reduce both hospital stay and costs, and late bradyarrhythmic events.
Regarding the TAVI procedure itself, operators may focus on decreasing the potential harm to the conduction system by adopting specific techniques to achieve high implantation depths. As an example, the use of transjugular intracardiac echocardiography, which allows direct visualization of the membranous septum, has been associated with a lower rate of PPI, as shown by Ishizu, et al.[92] On the other hand, a few studies explored the potential predictive effect of right atrial pacing during the procedure. Despite data from two studies that showed distinct results, atrial pacing has emerged as a tool to evaluate the subsequent risk for PPI.[93,94] Although there is potential interest, these strategies require additional central venous access and longer procedures, which may be at odds with the current minimalist approach in the TAVI setting.
The occurrence of TAVI-related CDs can be related to an acute, transient inflammation response triggered by the procedure. Thus, there is interest in exploring the potential use of anti-inflammatory agents in the periprocedural TAVI period to decrease the rate of new-onset CDs. However, retrospective, single-center studies did no demonstrate a reduction of PPI related to intraprocedural or post TAVI exposure to glucocorticoids.[95,96] The ongoing, prospective, randomized pivotal GLUCO-TAVR study (NCT06076824), will assess the efficacy of peri-procedure glucocorticoid treatment and will shed more light on this issue. In the same line, the randomized Co-STAR trial (NCT04870424) will study the use of colchicine in the periprocedural period and its impact on new-onset CDs. Moreover, this transitory nature may explain the observed early recovery of ECG-CDs following discharge in some patients with ECG-CDs, and the lack of significant ventricular pacing beyond the first weeks after TAVI. [97,98]
On the other hand, Chang, et al.[99] recently addressed the use of a temporary-permanent pacemaker (an active fixation lead with the pulse generator placed over the skin using an adhesive dressing) as a 1-month bridge to decision in patients with new-onset ECG-CDs. At 30 days, 53/70 (76%) of the included patients (of note, 77% of them suffered CHB following TAVI) did not have pacing indication and the temporary lead was removed with no PPI. Two adverse events related to the temporary active lead were reported, with no mortality among this group. This strategy confirmed a high rate of recovery within 1 month following the procedure and drastically reduced the rate of PPI.[99] These interesting results must be confirmed in larger cohorts and prospective, randomized data are needed.
There is still room for improvement on the use of EP studies before discharge to guide the management of patients with de novo ECG-CDs. Although European guidelines recommend PPI in patients with an HV interval of > 70 ms,[86] this threshold is extrapolated from previous data from studies not performed in TAVI patients. The potential transient nature of TAVI-related CDs may result in a risk of unnecessary treatment if the EP study is conducted shortly after TAVI. The ongoing “Reversibility of Cardiac Conduction Disturbances Following TAVI (TAVI-REVERSE)” study is a prospective, multicenter study that aims to include over 209 patients with a clinical need for EP study following TAVI (NCT06481137). For those who have a positive EP study, PPI will be carried out as per clinical guidelines. Of note, a second EP study will be repeated one month after the procedure in those patients with initial positive EP study. This will enable the assessment of conduction recovery rates and potential factors predicting it, leading to the proposal of new thresholds for PPI.
In recent years, several studies assessed the clinical use of ambulatory ECG monitoring in the context of TAVI, providing important insights into the arrhythmic burden of TAVI patients after discharge.[62,100–106] In summary, promising results have been obtained on the clinical impact of ambulatory ECG monitoring post-TAVI, with significant therapeutic changes such as PPI without a significant impact on safety. However, further data would be needed to confirm these findings and provide additional evidence on the usefulness and cost-effectiveness of AECG monitoring in TAVI recipients, which may entail a risk of overtreatment. In this line, randomized studies using AECG monitoring would provide significant data to evaluate its clinical and economic impact (e.g., sudden cardiac death, unplanned hospitalization, length of stay).
Since PPI and a high burden of right ventricular apical pacing (> 20%) is associated with worse clinical outcomes (mainly heart failure hospitalisations), new pacing modalities are now considered in these patients to prevent pacing induced cardiomyopathy. Physiological pacing using either the His bundle or the left bundle branch area pacing techniques is a promising avenue and clinical trials are underway. In some patients, the use of leadless pacing systems (single or dual chamber) can be an alternative to prevent complications associated with transvenous systems and early discharge.
Finally, the current recommendations regarding the management of TAVI-related CDs came from a non-validated expert consensus.[60] The study called “Prospective Validation of a Pre-specified Algorithm for the Management of Conduction Disturbances Following Transcatheter Aortic Valve Replacement (PROMOTE)” (NCT04139616) will enroll > 2000 patients that will follow the algorithm proposed in the consensus statement. This large-scale, multicenter, observational, prospective study will collect data from a large contemporary TAVI cohort that, for the first time, will follow a uniform post-procedure management. This upcoming study will provide significant data on several unmet needs, including periprocedural and late arrhythmic disorders, management of patients with new-onset LBBB or baseline RBBB, and the use of ambulatory ECG monitoring and EP studies. This study should help to identify subgroups with potential for improvement, which may impact hospital stay. Other significant upcoming studies in the field of new-onset CDs after TAVI are summarized in Table 1.
| NCT number | Study name | Population | N | Design | Intervention | Main outcomes |
| NCT04139616 | PROMOTE | All TAVI recipients without prior PPI | 2000 | Observational, prospective | Application of a pre-specified algorithm for the management of CDs post-TAVR | Implementation of the algorithm. Incidence of PPI and sudden cardiac death up to 1 year. |
| NCT06481137 | TAVI-REVERSE | TAVI patients with clinical EP study indication | 209 | Observational, Prospective | HV > 70 ms: PPI implantation and ambulatory ECG monitoring. Second EP at 1 month. HV < 70 ms: Ambulatory ECG monitoring. |
Evaluate the incidence of retrogradation of infra-Hisian conduction disturbance at 30-45 days following TAVI. |
| NCT06076824 | GLUCO-TAVR | All TAVI recipients without prior PPI | 100 | Prospective, randomized | Glucocorticoid administration vs placebo. | New-onset conduction disturbances. |
| NCT04870424 | Co-STAR | All TAVI recipients without prior PPI | 200 | Prospective, randomized | Colchicine administration vs placebo. | New-onset conduction disturbances and new-onset atrial fibrillation |
| NCT02659137 | HESITATE | All TAVI recipients without pre-existent CDs | 100 | Observational, prospective | EPS during the procedure. | Measurement of the HV interval upon occurrence of LBBB. |
| NCT04489095 | Conduction Disease After Transcatheter Aortic Valve Replacement | All TAVI recipients without prior PPI | 200 | Prospective, observational | EPS immediately before and after TAVI. | Correlation between delta values of EPS findings and high-grade conduction disturbances at 1 year. |
| NCT03303612 | COME TAVI | TAVR recipients with new-onset LBBB | 200 | Randomized, prospective. | Group 1: EPS-based strategy. Group 2: Clinical follow-up with implantable cardiac monitoring. |
Incidence of the composite of cardiovascular hospitalization, syncope or death at 1 year. Incidence of HAVB at 1 year. |
| NCT04482816 | PHYS-TAVI | TAVI recipients with HAVB pacing indication and LVEF > 50% | 24 | Randomized, prospective. | Experimental: Physiological (His system) pacing. Active Comparator: Right ventricular pacing. |
Composite of survival, NYHA improvement and >25% increase in the 6MWT at 1 year. LVEF at 1 year. |
| NCT05308888 | IMPACT | All TAVI recipients without prior PPI | 100 | Prospective, observational | Impact of Local Tissue Inflammation: Evaluation by PET | Occurrence of conduction disturbances |
| NCT05721170 | BETA-TAVI | All TAVI recipients with previous oral betablocker treatment | 347 | Prospective, randomized. | Beta-blockers continuation vs interruption. | Permanent pacemaker implantation. |
| NCT05278585 | PACE-TAVI | All TAVI recipients without prior PPI | 500 | Prospective, observational | Rapid atrial pacing during the procedure. | Permanent pacemaker implantation. |
| CDs: Conduction disturbances; EP study: electrophysiological study; HAVB: high-degree atrioventricular block; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; NYHA: New York heart association; 6-MWT: 6 min walking test; PET: Positron Emission Tomography; PPI: permanent pacemaker implantation. | ||||||
In conclusion, the occurrence of new-onset CDs following the procedure is still a major concern in the TAVI field. In the absence of non-arrhythmic complications, its management will definitely determine the duration of the hospitalization. However, while there is a current trend to shorten the post-procedural LOS, available evidence does not support applying this approach in high-risk groups such as those with baseline RBBB or new-onset CDs. Improvements in intraprocedural techniques, the use of ambulatory ECG monitoring and EP studies, and future data will provide more insight into resolving this challenging issue.
| [1] |
Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997; 29: 630−634.
|
| [2] |
Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368: 1005−1011. doi: 10.1016/S0140-6736(06)69208-8
|
| [3] |
Alain C, Helene E, Assaf B, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis. Circulation 2002; 106: 3006−3008. doi: 10.1161/01.CIR.0000047200.36165.B8
|
| [4] |
Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2021; 17: e1126−e1196.
|
| [5] |
Carroll JD, Mack MJ, Vemulapalli S, et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2020; 76: 2492−2516. doi: 10.1016/j.jacc.2020.09.595
|
| [6] |
Cahill TJ, Chen M, Hayashida K, et al. Transcatheter aortic valve implantation: current status and future perspectives. Eur Heart J 2018; 39: 2625−2634. doi: 10.1093/eurheartj/ehy244
|
| [7] |
Katherine H. Chau. Trends in Permanent Pacemaker Implantation after TAVR: An STS/ACC TVT Registry Analysis. Presented at Transcatheter Cardiovascular Therapeutics (TCT) 2023. San Francisco, CA, USA, October 2023.
|
| [8] |
Nickpreet Singh. Outcomes of Patients With New LBBB After TAVR: Insights From the NCDR STS/ACC TVT Registry. Presented at Transcatheter Cardiovascular Therapeutics (TCT) 2023. San Francisco, CA, USA, October 2023.
|
| [9] |
Wayangankar SA, Elgendy IY, Xiang Q, et al. Length of Stay After Transfemoral Transcatheter Aortic Valve Replacement: An Analysis of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. JACC Cardiovasc Interv 2019; 12: 422−430. doi: 10.1016/j.jcin.2018.11.015
|
| [10] |
Perdoncin E, Greenbaum AB, Grubb KJ, et al. Safety of same-day discharge after uncomplicated, minimalist transcatheter aortic valve replacement in the COVID-19 era. Catheter Cardiovasc Interv 2021; 97: 940−947. doi: 10.1002/ccd.29453
|
| [11] |
Ooms JF, Cornelis K, Wijeysundera HC, et al. Safety and feasibility of early discharge after transcatheter aortic valve implantation with ACURATE Neo-the POLESTAR trial. Clin Res Cardiol 2025; 114: 341−349. doi: 10.1007/s00392-024-02436-z
|
| [12] |
Kooistra NHM, van Mourik MS, Rodríguez-Olivares R, et al. Late onset of new conduction disturbances requiring permanent pacemaker implantation following TAVI. Heart 2020; 106: 1244−1251. doi: 10.1136/heartjnl-2019-315967
|
| [13] |
Mangieri A, Lanzillo G, Bertoldi L, et al. Predictors of advanced conduction disturbances requiring a late (≥ 48 h) permanent pacemaker following transcatheter aortic valve replacement. JACC Cardiovasc Interv 2018; 11: 1519−1526. doi: 10.1016/j.jcin.2018.06.014
|
| [14] |
Jørgensen TH, De Backer O, Gerds TA, et al. Immediate Post-Procedural 12-Lead Electrocardiography as Predictor of Late Conduction Defects After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2018; 11: 1509−1518. doi: 10.1016/j.jcin.2018.04.011
|
| [15] |
Mazzella AJ, Hendrickson MJ, Arora S, et al. Shifting Trends in Timing of Pacemaker Implantation After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2021; 14: 232−234. doi: 10.1016/j.jcin.2020.09.034
|
| [16] |
Lilly SM, Deshmukh AJ, Epstein AE, et al. 2020 ACC Expert Consensus Decision Pathway on Management of Conduction Disturbances in Patients Undergoing Transcatheter Aortic Valve Replacement: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2020; 76: 2391−2411. doi: 10.1016/j.jacc.2020.08.050
|
| [17] |
Muntané-Carol G, Guimaraes L, Ferreira-Neto AN, et al. How does new-onset left bundle branch block affect the outcomes of transcatheter aortic valve repair? Expert Rev Med Devices 2019;16: 589–602.
|
| [18] |
Moreno R, Dobarro D, López de Sá E, et al. Cause of complete atrioventricular block after percutaneous aortic valve implantation: insights from a necropsy study. Circulation 2009; 120: e29−30.
|
| [19] |
Rubín JM, Avanzas P, del Valle R, et al. Atrioventricular conduction disturbance characterization in transcatheter aortic valve implantation with the CoreValve prosthesis. Circ Cardiovasc Interv 2011; 4: 280−286. doi: 10.1161/CIRCINTERVENTIONS.111.961649
|
| [20] |
Auffret V, Puri R, Urena M, et al. Conduction Disturbances After Transcatheter Aortic Valve Replacement: Current Status and Future Perspectives. Circulation 2017; 136: 1049−1069. doi: 10.1161/CIRCULATIONAHA.117.028352
|
| [21] |
Walther T, Manoharan G, Linke A, et al. Incidence of new-onset left bundle branch block and predictors of new permanent pacemaker following transcatheter aortic valve replacement with the PorticoTM valve. Eur J Cardiothorac Surg 2018; 54: 467−474. doi: 10.1093/ejcts/ezy078
|
| [22] |
Zaid S, Attizzani GF, Krishnamoorthy P, et al. First-in-Human Multicenter Experience of the Newest Generation Supra-Annular Self-Expanding Evolut FX TAVR System. JACC Cardiovasc Interv 2023; 16: 1626−1635.
|
| [23] |
Sammour YM, Lak H, Chahine J, et al. Clinical and echocardiographic outcomes with new-onset left bundle branch block after SAPIEN-3 transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2023; 101: 187−196. doi: 10.1002/ccd.30488
|
| [24] |
Pellegrini C, Garot P, Morice M-C, et al. Permanent pacemaker implantation and left bundle branch block with self-expanding valves - a SCOPE 2 subanalysis. EuroIntervention 2023; 18: e1077−e1087. doi: 10.4244/EIJ-D-22-00558
|
| [25] |
Baumbach A, van Royen N, Amat-Santos IJ, et al. LANDMARK comparison of early outcomes of newer-generation Myval transcatheter heart valve series with contemporary valves (Sapien and Evolut) in real-world individuals with severe symptomatic native aortic stenosis: a randomised non-inferiority trial. Lancet 2024; 403: 2695−2708. doi: 10.1016/S0140-6736(24)00821-3
|
| [26] |
Regueiro A, Abdul-Jawad Altisent O, Del Trigo M, et al. Impact of New-Onset Left Bundle Branch Block and Periprocedural Permanent Pacemaker Implantation on Clinical Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Circ Cardiovasc Interv 2016; 9: e003635. doi: 10.1161/CIRCINTERVENTIONS.115.003635
|
| [27] |
Ando T, Takagi H, ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. The Prognostic Impact of New-Onset Persistent Left Bundle Branch Block Following Transcatheter Aortic Valve Implantation: A Meta-analysis. Clin Cardiol 2016; 39: 544−550. doi: 10.1002/clc.22567
|
| [28] |
Urena M, Webb JG, Eltchaninoff H, et al. Late cardiac death in patients undergoing transcatheter aortic valve replacement: incidence and predictors of advanced heart failure and sudden cardiac death. J Am Coll Cardiol 2015; 65: 437−448. doi: 10.1016/j.jacc.2014.11.027
|
| [29] |
Toggweiler S, Stortecky S, Holy E, et al. The Electrocardiogram After Transcatheter Aortic Valve Replacement Determines the Risk for Post-Procedural High-Degree AV Block and the Need for Telemetry Monitoring. JACC Cardiovasc Interv 2016; 9: 1269−1276. doi: 10.1016/j.jcin.2016.03.024
|
| [30] |
Houthuizen P, van der Boon RMA, Urena M, et al. Occurrence, fate and consequences of ventricular conduction abnormalities after transcatheter aortic valve implantation. EuroIntervention 2014; 9: 1142−1150. doi: 10.4244/EIJV9I10A194
|
| [31] |
Urena M, Webb JG, Cheema A, et al. Impact of new-onset persistent left bundle branch block on late clinical outcomes in patients undergoing transcatheter aortic valve implantation with a balloon-expandable valve. JACC Cardiovasc Interv 2014; 7: 128−136. doi: 10.1016/j.jcin.2013.08.015
|
| [32] |
Carrabba N, Valenti R, Migliorini A, et al. Impact on Left Ventricular Function and Remodeling and on 1-Year Outcome in Patients With Left Bundle Branch Block After Transcatheter Aortic Valve Implantation. Am J Cardiol 2015; 116: 125−131. doi: 10.1016/j.amjcard.2015.03.054
|
| [33] |
Schymik G, Tzamalis P, Bramlage P, et al. Clinical impact of a new left bundle branch block following TAVI implantation: 1-year results of the TAVIK cohort. Clin Res Cardiol 2014; 104: 351−362.
|
| [34] |
Chamandi C, Barbanti M, Munoz-Garcia A, et al. Long-Term Outcomes in Patients With New-Onset Persistent Left Bundle Branch Block Following TAVR. JACC Cardiovasc Interv 2019; 12: 1175−1184. doi: 10.1016/j.jcin.2019.03.025
|
| [35] |
Nazif TM, Chen S, George I, et al. New-onset left bundle branch block after transcatheter aortic valve replacement is associated with adverse long-term clinical outcomes in intermediate-risk patients: an analysis from the PARTNER II trial. Eur Heart J 2019; 40: 2218−2227. doi: 10.1093/eurheartj/ehz227
|
| [36] |
Jørgensen TH, De Backer O, Gerds TA, et al. Mortality and Heart Failure Hospitalization in Patients With Conduction Abnormalities After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2019; 12: 52−61.
|
| [37] |
Faroux L, Chen S, Muntané-Carol G, et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: a systematic review and meta-analysis. Eur Heart J 2020; 41: 2771−2781. doi: 10.1093/eurheartj/ehz924
|
| [38] |
Reardon MJ, Chehab B, Smith D, et al. 30-Day Clinical outcomes of a self-expanding transcatheter aortic valve: the international PORTICO NG Study. JACC Cardiovasc Interv 2023; 16: 681−689. doi: 10.1016/j.jcin.2023.02.002
|
| [39] |
Yamamoto M, Yashima F, Shirai S, et al. Performance and outcomes of the SAPIEN 3 Ultra RESILIA transcatheter heart valve in the OCEAN-TAVI registry. EuroIntervention 2024; 20: 579−590. doi: 10.4244/EIJ-D-23-00996
|
| [40] |
Thiele H, Kurz T, Feistritzer HJ, et al. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: the randomized SOLVE-TAVI trial. Eur Heart J 2020; 41: 1890−1899.
|
| [41] |
Herrmann HC, Mehran R, Blackman DJ, et al. Self-Expanding or Balloon-Expandable TAVR in Patients with a Small Aortic Annulus. N Engl J Med 2024; 390: 1959−1971. doi: 10.1056/NEJMoa2312573
|
| [42] |
Curtis AB, Worley SJ, Adamson PB, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med 2013; 368: 1585−1593. doi: 10.1056/NEJMoa1210356
|
| [43] |
Shen L, Jhund PS, Docherty KF, et al. Prior Pacemaker Implantation and Clinical Outcomes in Patients With Heart Failure and Preserved Ejection Fraction. JACC Heart Fail 2019; 7: 418−427. doi: 10.1016/j.jchf.2018.12.006
|
| [44] |
Elder DHJ, Lang CC, Choy AM. Pacing-induced heart disease: understanding the pathophysiology and improving outcomes. Expert Rev Cardiovasc Ther 2011; 9: 877−886. doi: 10.1586/erc.11.82
|
| [45] |
Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003; 107: 2932−2937. doi: 10.1161/01.CIR.0000072769.17295.B1
|
| [46] |
Rao K, Chan B, Baer A, et al. A Systematic Review of Delayed High-Grade Atrioventricular Block After Transcatheter Aortic Valve Implantation. CJC Open 2024; 6: 86−95. doi: 10.1016/j.cjco.2023.10.003
|
| [47] |
Vendrik J, Vlastra W, van Mourik MS, et al. Early mobilisation after transfemoral transcatheter aortic valve implantation: results of the MobiTAVI trial. Neth Heart J 2020; 28: 240−248. doi: 10.1007/s12471-020-01374-5
|
| [48] |
Lauck SB, Wood DA, Baumbusch J, et al. Vancouver transcatheter aortic valve replacement clinical pathway: minimalist approach, standardized care, and discharge criteria to reduce length of stay. Circ Cardiovasc Qual Outcomes 2016; 9: 312−321. doi: 10.1161/CIRCOUTCOMES.115.002541
|
| [49] |
Barbanti M, Capranzano P, Ohno Y, et al. Early discharge after transfemoral transcatheter aortic valve implantation. Heart 2015; 101: 1485−1490. doi: 10.1136/heartjnl-2014-307351
|
| [50] |
Aldalati O, Keshavarzi F, Kaura A, et al. Factors associated with safe early discharge after transcatheter aortic valve implantation. Cardiol J 2018; 25: 14−23. doi: 10.5603/CJ.a2017.0087
|
| [51] |
Alkhalil A, Lamba H, Deo S, et al. Safety of shorter length of hospital stay for patients undergoing minimalist transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2018; 91: 345−353. doi: 10.1002/ccd.27230
|
| [52] |
Mallikethi-Reddy S, Akintoye E, Telila T, et al. Transcatheter aortic valve implantation in the United States: Predictors of early hospital discharge. J Interv Cardiol 2017; 30: 149−155. doi: 10.1111/joic.12373
|
| [53] |
Kamioka N, Wells J, Keegan P, et al. Predictors and clinical outcomes of next-day discharge after minimalist transfemoral transcatheter aortic valve replacement. JACC Cardiovasc Interv 2018; 11: 107−115. doi: 10.1016/j.jcin.2017.10.021
|
| [54] |
Wood DA, Lauck SB, Cairns JA, et al. The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) Clinical Pathway Facilitates Safe Next-Day Discharge Home at Low-, Medium-, and High-Volume Transfemoral Transcatheter Aortic Valve Replacement Centers: The 3M TAVR Study. JACC Cardiovasc Interv 2019; 12: 459−469. doi: 10.1016/j.jcin.2018.12.020
|
| [55] |
Lauck SB, Sathananthan J, Park J, et al. Post-procedure protocol to facilitate next-day discharge: Results of the multidisciplinary, multimodality but minimalist TAVR study. Catheter Cardiovasc Interv 2020; 96: 450−458. doi: 10.1002/ccd.28617
|
| [56] |
Barbanti M, Baan J, Spence MS, et al. Feasibility and safety of early discharge after transfemoral transcatheter aortic valve implantation - rationale and design of the FAST-TAVI registry. BMC Cardiovasc Disord 2017; 17: 259. doi: 10.1186/s12872-017-0693-0
|
| [57] |
Barbanti M, van Mourik MS, Spence MS, et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: the multicentre European FAST-TAVI trial. EuroIntervention 2019; 15: 147−154. doi: 10.4244/EIJ-D-18-01197
|
| [58] |
Durand E, Beziau-Gasnier D, Michel M, et al. Reducing length of stay after transfemoral transcatheter aortic valve implantation: the FAST-TAVI II trial. Eur Heart J 2024; 45: 952−962. doi: 10.1093/eurheartj/ehae081
|
| [59] |
Cerrato E, Nombela-Franco L, Nazif TM, et al. Evaluation of current practices in transcatheter aortic valve implantation: The WRITTEN (WoRldwIde TAVI ExperieNce) survey. Int J Cardiol 2017; 228: 640−647. doi: 10.1016/j.ijcard.2016.11.104
|
| [60] |
Rodés-Cabau J, Ellenbogen KA, Krahn AD, et al. Management of Conduction Disturbances Associated With Transcatheter Aortic Valve Replacement: JACC Scientific Expert Panel. J Am Coll Cardiol 2019; 74: 1086−1106. doi: 10.1016/j.jacc.2019.07.014
|
| [61] |
Muntané-Carol G, Alméndarez M, Junquera L, et al. Long-Term Electrocardiographic Changes and Clinical Outcomes of Transcatheter Aortic Valve Implantation Recipients Without New Postprocedural Conduction Disturbances. Am J Cardiol 2020; 125: 107−113. doi: 10.1016/j.amjcard.2019.09.047
|
| [62] |
Muntané-Carol G, Okoh AK, Chen C, et al. Ambulatory Electrocardiographic Monitoring Following Minimalist Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2021; 14: 2711−2722. doi: 10.1016/j.jcin.2021.08.039
|
| [63] |
Jilaihawi H, Zhao Z, Du R, et al. Minimizing Permanent Pacemaker Following Repositionable Self-Expanding Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2019; 12: 1796−1807. doi: 10.1016/j.jcin.2019.05.056
|
| [64] |
Sá MP, Van den Eynde J, Jacquemyn X, et al. Cusp-overlap versus coplanar view in transcatheter aortic valve implantation with self-expandable valves: A meta-analysis of comparative studies. Catheter Cardiovasc Interv 2023; 101: 639−650. doi: 10.1002/ccd.30562
|
| [65] |
Ishizu K, Shirai S, Kawaguchi T, et al. Effect of Radiolucent Line-Guided Balloon-Expandable Transcatheter Aortic Valve Implantation on Subsequent Pacemaker Rate. Am J Cardiol 2022; 165: 72−80. doi: 10.1016/j.amjcard.2021.11.010
|
| [66] |
Akodad M, Blanke P, Nestelberger T, et al. Hybrid Approach Using the Cusp-Overlap Technique for Transcatheter Aortic Valve Replacement With a Balloon-Expandable Valve. JACC Cardiovasc Interv 2022; 15: 2387−2395. doi: 10.1016/j.jcin.2022.10.035
|
| [67] |
Ochiai T, Yamanaka F, Shishido K, et al. Impact of High Implantation of Transcatheter Aortic Valve on Subsequent Conduction Disturbances and Coronary Access. JACC Cardiovasc Interv 2023; 16: 1192−1204. doi: 10.1016/j.jcin.2023.03.021
|
| [68] |
Ravaux JM, Di Mauro M, Vernooy K, et al. One-year pacing dependency after pacemaker implantation in patients undergoing transcatheter aortic valve implantation: Systematic review and meta-analysis. JTCVS Open 2021; 6: 41−55. e15.
|
| [69] |
Muntané-Carol G, Del Val D, Junquera L, et al. Timing and evolution of advanced conduction disturbances in patients with right bundle branch block undergoing transcatheter aortic valve replacement. Europace 2020; 22: 1537−1546. doi: 10.1093/europace/euaa149
|
| [70] |
Nuche J, Masso van-Roessel A, Nault I, et al. Temporary active fixation lead pacemaker in transcatheter aortic valve replacement patients with right bundle branch block. Heart Rhythm 2023; 20: 309−310. doi: 10.1016/j.hrthm.2022.09.007
|
| [71] |
Seder-Colomina E, Maille B, Klein V, et al. Active fixation lead temporary pacing in patients with right bundle block undergoing transcatheter aortic valve implantation. Arch Cardiovasc Dis 2023; 116: 291−293. doi: 10.1016/j.acvd.2023.02.001
|
| [72] |
Kassier A, Velagapudi P, Shrestha NM, et al. Optimizing Care of Patients With Right Bundle Branch Block Undergoing Transcatheter Aortic Valve Replacement. Cardiovasc Revasc Med 2022; 42: 17−25. doi: 10.1016/j.carrev.2022.03.018
|
| [73] |
Wolfe RR, Driscoll DJ, Gersony WM, et al. Arrhythmias in patients with valvar aortic stenosis, valvar pulmonary stenosis, and ventricular septal defect. Results of 24-hour ECG monitoring. Circulation 1993; 87: I89−101.
|
| [74] |
MacMillan RM, Demorizi NM, Gessman LJ, et al. Correlates of prolonged HV conduction in aortic stenosis. Am Heart J 1985; 110: 56−60. doi: 10.1016/0002-8703(85)90514-9
|
| [75] |
Urena M, Hayek S, Cheema AN, et al. Arrhythmia burden in elderly patients with severe aortic stenosis as determined by continuous electrocardiographic recording: toward a better understanding of arrhythmic events after transcatheter aortic valve replacement. Circulation 2015; 131: 469−477. doi: 10.1161/CIRCULATIONAHA.114.011929
|
| [76] |
Winter JL, Healey JS, Sheth TN, et al. Remote Ambulatory Cardiac Monitoring Before and After Transcatheter Aortic Valve Replacement. CJC open 2020; 2: 416−419. doi: 10.1016/j.cjco.2020.04.006
|
| [77] |
Asmarats L, Nault I, Ferreira-Neto AN, et al. Prolonged Continuous Electrocardiographic Monitoring Prior to Transcatheter Aortic Valve Replacement: The PARE Study. JACC Cardiovasc Interv 2020; 13: 1763−1773. doi: 10.1016/j.jcin.2020.03.031
|
| [78] |
Schoechlin S, Eichenlaub M, Müller-Edenborn B, et al. Risk Stratification for Pacemaker Implantation after Transcatheter Aortic Valve Implantation in Patients with Right Bundle Branch Block. J Clin Med 2022; 11: 5580. doi: 10.3390/jcm11195580
|
| [79] |
Sugiyama Y, Miyashita H, Yokoyama H, et al. Risk Assessment of Permanent Pacemaker Implantation After Transcatheter Aortic Valve Implantation in Patients With Preexisting Right Bundle Branch Block. Am J Cardiol 2024; 213: 151−160. doi: 10.1016/j.amjcard.2023.12.004
|
| [80] |
Tovia-Brodie O, Letourneau-Shesaf S, Hochstadt A, et al. The Utility of Prophylactic Pacemaker Implantation in Right Bundle Branch Block Patients Pre-Transcatheter Aortic Valve Implantation. Isr Med Assoc J 2019; 21: 790−795.
|
| [81] |
Pavitt C, Waleed M, Arunothayaraj S, et al. Transcatheter Aortic Valve Implantation in Patients With Right Bundle-Branch Block: Should Prophylactic Pacing Be Undertaken? J Invasive Cardiol 2023; 35: E37–E45.
|
| [82] |
Fukutomi M, Hokken T, Wong I, et al. Prophylactic Permanent Pacemaker Implantation in Patients With Right Bundle Branch Block Undergoing TAVR. JACC Cardiovasc Interv 2021; 14: 1272−1274. doi: 10.1016/j.jcin.2021.03.043
|
| [83] |
Fukutomi M, Hokken T, Wong I, et al. Prophylactic permanent pacemaker strategy in patients with right bundle branch block undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2021; 98: E1017−E1025.
|
| [84] |
Husser O, Pellegrini C, Kim W-K, et al. Transcatheter Valve SELECTion in Patients With Right Bundle Branch Block and Impact on Pacemaker Implantations. JACC Cardiovasc Interv 2019; 12: 1781−1793. doi: 10.1016/j.jcin.2019.05.055
|
| [85] |
Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019; 74: e51−e156. doi: 10.1016/j.jacc.2018.10.044
|
| [86] |
Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021; 42: 3427−3520. doi: 10.1093/eurheartj/ehab364
|
| [87] |
Massoullié G, Ploux S, Souteyrand G, et al. Incidence and management of atrioventricular conduction disorders in new-onset left bundle branch block after TAVI: A prospective multicenter study. Heart Rhythm 2023; 20: 699−706. doi: 10.1016/j.hrthm.2023.01.013
|
| [88] |
Rodés-Cabau J, Urena M, Nombela-Franco L, et al. Arrhythmic Burden as Determined by Ambulatory Continuous Cardiac Monitoring in Patients With New-Onset Persistent Left Bundle Branch Block Following Transcatheter Aortic Valve Replacement: The MARE Study. JACC Cardiovasc Interv 2018; 11: 1495−1505. doi: 10.1016/j.jcin.2018.04.016
|
| [89] |
Faroux L, Muntané-Carol G, Urena M, et al. Late Electrocardiographic Changes in Patients With New-Onset Left Bundle Branch Block Following Transcatheter Aortic Valve Implantation. Am J Cardiol 2020; 125: 795−802. doi: 10.1016/j.amjcard.2019.11.025
|
| [90] |
Muntané-Carol G, Portero-Portaz JJ, Alméndarez M, et al. Persistent Intraprocedural Atrioventricular Block in Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2021; 14: 1502−1503. doi: 10.1016/j.jcin.2021.04.020
|
| [91] |
Nuche J, Ellenbogen KA, Mittal S, et al. Conduction Disturbances After Transcatheter Aortic Valve Replacement: An Update on Epidemiology, Preventive Strategies, and Management. JACC Cardiovasc Interv 2024 Nov 25;17: 2575−2595.
|
| [92] |
Ishizu K, Shirai S, Miyawaki N, et al. Impact of Transjugular Intracardiac Echocardiography-Guided Self-Expandable Transcatheter Aortic Valve Implantation on Reduction of Conduction Disturbances. Circ Cardiovasc Interv 2024; 17: e013094.
|
| [93] |
Krishnaswamy A, Sammour Y, Mangieri A, et al. The Utility of Rapid Atrial Pacing Immediately Post-TAVR to Predict the Need for Pacemaker Implantation. JACC Cardiovasc Interv 2020; 13: 1046−1054. doi: 10.1016/j.jcin.2020.01.215
|
| [94] |
Tan BE-X, Hashem A, Boppana LKT, et al. Utility of rapid atrial pacing before and after TAVR with balloon-expandable valve in predicting permanent pacemaker implantation. Catheter Cardiovasc Interv 2023; 102: 919−928. doi: 10.1002/ccd.30817
|
| [95] |
Barone L, Muscoli S, Belli M, et al. Effect of acute CORticosteroids on conduction defects after Transcatheter Aortic Valve Implantation: the CORTAVI study. J Cardiovasc Med (Hagerstown) 2023; 24: 676−679.
|
| [96] |
Tiago C, Dias Vaz M, Marques A, et al. Intraoperative Corticosteroids and Pacemaker Implantation After Transcatheter Aortic Valve Replacement. Cureus 2024; 16: e56824.
|
| [97] |
Costa G, Zappulla P, Barbanti M, et al. Pacemaker Dependency after Transcatheter Aortic Valve Implantation: Incidence, Predictors and Long-term outcomes. EuroIntervention 2019; 15: 875−883. doi: 10.4244/EIJ-D-18-01060
|
| [98] |
Meduri CU, Kereiakes DJ, Rajagopal V, et al. Pacemaker Implantation and Dependency After Transcatheter Aortic Valve Replacement in the REPRISE III Trial. J Am Heart Assoc 2019; 8: e012594. doi: 10.1161/JAHA.119.012594
|
| [99] |
Chang S, Jiang Z, Liu X, et al. Permanent pacemaker reduction using temporary-permanent pacemaker as a 1-month bridge after transcatheter aortic valve replacement: a prospective, multicentre, single-arm, observational study. EClinicalMedicine 2024; 72: 102603. doi: 10.1016/j.eclinm.2024.102603
|
| [100] |
Beccarino N, Epstein LM, Khodak A, et al. The utility and impact of outpatient telemetry monitoring in post-transcatheter aortic valve replacement patients. Cardiovasc Revasc Med 2024; 64: 15−20.
|
| [101] |
Natarajan MK, Sheth TN, Wijeysundera HC, et al. Remote ECG monitoring to reduce complications following transcatheter aortic valve implantations: the Redirect TAVI study. Europace 2022; 24: 1475−1483. doi: 10.1093/europace/euac042
|
| [102] |
Fan J, Dai H, Guo Y, et al. Smartwatch-Detected Arrhythmias in Patients After Transcatheter Aortic Valve Replacement (TAVR): Analysis of the SMART TAVR Trial. J Med Internet Res 2024; 26: e41843. doi: 10.2196/41843
|
| [103] |
Reiter C, Lambert T, Kellermair J, et al. Delayed Total Atrioventricular Block After Transcatheter Aortic Valve Replacement Assessed by Implantable Loop Recorders. JACC Cardiovasc Interv 2021; 14: 2723−2732. doi: 10.1016/j.jcin.2021.09.003
|
| [104] |
Tarakji KG, Patel D, Krishnaswamy A, et al. Bradyarrhythmias detected by extended rhythm recording in patients undergoing transcatheter aortic valve replacement (Brady-TAVR Study). Heart Rhythm 2022; 19: 381−388. doi: 10.1016/j.hrthm.2021.11.020
|
| [105] |
Tian Y, Padmanabhan D, McLeod CJ, et al. Utility of 30-Day Continuous Ambulatory Monitoring to Identify Patients With Delayed Occurrence of Atrioventricular Block After Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv 2019; 12: e007635. doi: 10.1161/CIRCINTERVENTIONS.118.007635
|
| [106] |
Ream K, Sandhu A, Valle J, et al. Ambulatory Rhythm Monitoring to Detect Late High-Grade Atrioventricular Block Following Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2019; 73: 2538−2547.
|
| [1] | Abbas Alshami, Carlos Romero, America Avila, Joseph Varon. Management of Hypertensive Crises in the Elderly[J]. Journal of Geriatric Cardiology, 2018, 15(7): 504-512. DOI: 10.11909/j.issn.1671-5411.2018.07.007 |
| [2] | Eiji Ichimoto, Adam Arnofsky, Michael Wilderman, Richard Goldweit, Joseph De Gregorio. Early mortality and safety after transcatheter aortic valve replacement using the SAPIEN 3 in nonagenarians[J]. Journal of Geriatric Cardiology, 2018, 15(6): 387-393. DOI: 10.11909/j.issn.1671-5411.2018.06.002 |
| [3] | Panagiotis Karyofillis, Anna Kostopoulou, Sofia Thomopoulou,, Martha Habibi, Efthimios Livanis, George Karavolias, Vassilis Voudris. Conduction abnormalities after transcatheter aortic valve implantation[J]. Journal of Geriatric Cardiology, 2018, 15(1): 105-112. DOI: 10.11909/j.issn.1671-5411.2018.01.004 |
| [4] | Emmanouil Chourdakis, Ioanna Koniari, George Hahalis, Nicholas G Kounis, Karl Eugen Hauptmann. Early prosthetic valve endocarditis after transcatheter aortic valve implan-tation with periannular complication[J]. Journal of Geriatric Cardiology, 2017, 14(11): 711-711. DOI: 10.11909/j.issn.1671-5411.2017.11.007 |
| [5] | Lyudmila Sergeevna Korostovtseva, Nadezhda Edvinovna Zvartau, Oxana Petrovna Rotar, Yurii Vladimirovich Sviryaev, Aleksandra Olegovna Konradi. Predictors of heart rhythm disturbances in hypertensive obese patients with obstructive sleep apnea[J]. Journal of Geriatric Cardiology, 2017, 14(9): 553-562. DOI: 10.11909/j.issn.1671-5411.2017.09.010 |
| [6] | Shi-Jian CHEN, Wei LIU, Bao-Tao HUANG, Jia-Yu TSAUO, Xiao-Bo PU, Yong PENG, Mao CHEN, De-Jia HUANG. The impact of optimal medical therapy at discharge on mortality in patients with coronary artery disease[J]. Journal of Geriatric Cardiology, 2017, 14(2): 100-107. DOI: 10.11909/j.issn.1671-5411.2017.02.004 |
| [7] | Gary Tse. Novel conduction-repolarization indices for the stratification of arrhythmic risk[J]. Journal of Geriatric Cardiology, 2016, 13(9): 811-812. DOI: 10.11909/j.issn.1671-5411.2016.09.008 |
| [8] | András Vereckei, Gábor Katona, Zsuzsanna Szelényi, Gábor Szénási, Bálint Kozman, István Karádi. The role of electrocardiography in the elaboration of a new paradigm in cardiac resynchronization therapy for patients with nonspecific intraventricular conduction disturbance[J]. Journal of Geriatric Cardiology, 2016, 13(2): 118-125. DOI: 10.11909/j.issn.1671-5411.2016.02.002 |
| [9] | Jong Shin Woo, Tae-Kyung Yu, Woo-Shik Kim, Kwon Sam Kim, Weon Kim. Early prediction of myocardial viability after acute myocardial infarction by two-dimensional speckle tracking imaging[J]. Journal of Geriatric Cardiology, 2015, 12(5): 474-481. DOI: 10.11909/j.issn.1671-5411.2015.05.002 |
| [10] | Mehmet Tezcan, Omer Yiginer, Mehmet Dogan, Gokhan Degirmencioglu. A step forward parameter for the effects of transcatheter aortic valve implan-tation: transmural dispersion of repolarization[J]. Journal of Geriatric Cardiology, 2015, 12(3): 326-328. DOI: 10.11909/j.issn.1671-5411.2015.03.012 |
| NCT number | Study name | Population | N | Design | Intervention | Main outcomes |
| NCT04139616 | PROMOTE | All TAVI recipients without prior PPI | 2000 | Observational, prospective | Application of a pre-specified algorithm for the management of CDs post-TAVR | Implementation of the algorithm. Incidence of PPI and sudden cardiac death up to 1 year. |
| NCT06481137 | TAVI-REVERSE | TAVI patients with clinical EP study indication | 209 | Observational, Prospective | HV > 70 ms: PPI implantation and ambulatory ECG monitoring. Second EP at 1 month. HV < 70 ms: Ambulatory ECG monitoring. |
Evaluate the incidence of retrogradation of infra-Hisian conduction disturbance at 30-45 days following TAVI. |
| NCT06076824 | GLUCO-TAVR | All TAVI recipients without prior PPI | 100 | Prospective, randomized | Glucocorticoid administration vs placebo. | New-onset conduction disturbances. |
| NCT04870424 | Co-STAR | All TAVI recipients without prior PPI | 200 | Prospective, randomized | Colchicine administration vs placebo. | New-onset conduction disturbances and new-onset atrial fibrillation |
| NCT02659137 | HESITATE | All TAVI recipients without pre-existent CDs | 100 | Observational, prospective | EPS during the procedure. | Measurement of the HV interval upon occurrence of LBBB. |
| NCT04489095 | Conduction Disease After Transcatheter Aortic Valve Replacement | All TAVI recipients without prior PPI | 200 | Prospective, observational | EPS immediately before and after TAVI. | Correlation between delta values of EPS findings and high-grade conduction disturbances at 1 year. |
| NCT03303612 | COME TAVI | TAVR recipients with new-onset LBBB | 200 | Randomized, prospective. | Group 1: EPS-based strategy. Group 2: Clinical follow-up with implantable cardiac monitoring. |
Incidence of the composite of cardiovascular hospitalization, syncope or death at 1 year. Incidence of HAVB at 1 year. |
| NCT04482816 | PHYS-TAVI | TAVI recipients with HAVB pacing indication and LVEF > 50% | 24 | Randomized, prospective. | Experimental: Physiological (His system) pacing. Active Comparator: Right ventricular pacing. |
Composite of survival, NYHA improvement and >25% increase in the 6MWT at 1 year. LVEF at 1 year. |
| NCT05308888 | IMPACT | All TAVI recipients without prior PPI | 100 | Prospective, observational | Impact of Local Tissue Inflammation: Evaluation by PET | Occurrence of conduction disturbances |
| NCT05721170 | BETA-TAVI | All TAVI recipients with previous oral betablocker treatment | 347 | Prospective, randomized. | Beta-blockers continuation vs interruption. | Permanent pacemaker implantation. |
| NCT05278585 | PACE-TAVI | All TAVI recipients without prior PPI | 500 | Prospective, observational | Rapid atrial pacing during the procedure. | Permanent pacemaker implantation. |
| CDs: Conduction disturbances; EP study: electrophysiological study; HAVB: high-degree atrioventricular block; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; NYHA: New York heart association; 6-MWT: 6 min walking test; PET: Positron Emission Tomography; PPI: permanent pacemaker implantation. | ||||||