| Citation: | Please cite this article as: Cespón-Fernández M, Escalona-Canal EJ, Sánchez-Ramos J, Raposeiras-Roubín S, Abdulkader-Sande S, Cobas-Paz RJ, Torreira-Banzas C, Abu-Assi E, Teijeira-Bautista S, Domínguez-Aristegui P, García-Pavía P, Escalona-Canal ME, Cespón-Outeda E, Ortiz-Rey JA. Amyloid deposits in prostate biopsy as an opportunity to diagnose early cardiac amyloidosis. J Geriatr Cardiol 2025; 22(1): 169−177. DOI: 10.26599/1671-5411.2025.01.007. |
The diagnostic delay of cardiac amyloidosis (CA) is known to be substantially long. A prolonged time from symptoms onset to diagnosis negatively impacts quality of life and life expectancy of the affected patients. We aim to describe the role of the incidental finding of amyloid deposits in prostatic tissue as an early marker of CA.
A systematic cardiological evaluation, comprising ECG, echocardiogram and 99mTc-DPD scintigraphy, was offered to a cohort of 19 patients with incidental prostatic amyloidosis (PA) findings, propectively detected between 2014–2023, to assess cardiac involvement.
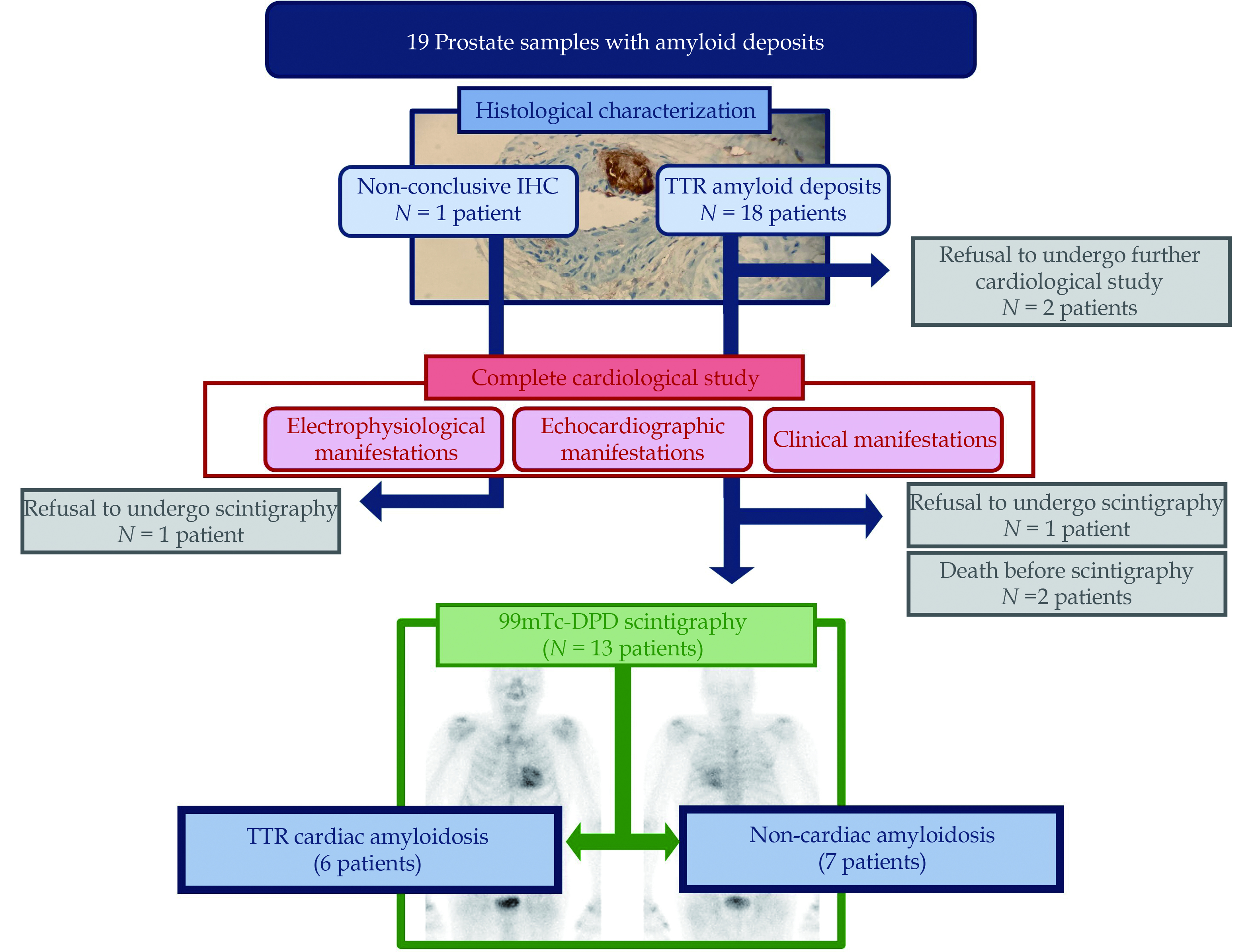
The median age of the patients was 80.2 years (IQR: 74.9 –82.6 years). Histopathological study revealed amyloid deposits within the walls of small vessels (predominantly small arteries) in 18 patients and mainly in the stroma in the remaining case. All of them were immunohistochemically positive for transthyretin (ATTR) except one patient, with known myeloma, which was unconclusive fo ATTR. Clonal dyscrasia was excluded in the rest of the patients. Thirteen patients (68.4%) underwent all cardiological tests, 4 patients (21.1%) underwent only ECG and echocardiographic evaluation and two patients (10.5%) refused to undergo any cardiological study. Among 13 individuals undergoing the complete evaluation, six patients were eventually diagnosed with CA (46.15%). All of them were asymptomatic from a cardiovascular point of view at the time of the prostate biopsy.
The finding of PA should prompt a complete cardiovascular examination, given the significant percentage of patients eventually diagnosed with early-stage CA. Multidisciplinary collaboration among different medical specialists must be encouraged, given the potential clinical impact of CA early diagnosis.
Amyloidosis is caused by an abnormal extracellular deposition of insoluble misfolded proteins (amyloid fibrils) in a variety of organs, causing a progressive dysfunction. Cardiac involvement in amyloidosis is the consequence of the accumulation of these amyloid fibrils in the myocardial interstitial space between myocytes, leading to impaired compliance and, eventually, heart failure. There are different types of cardiac amyloidosis (CA), depending on the accumulated amyloidogenic proteins. The vast majority of them results from the deposition of monoclonal immunoglobulin light chains (AL) or transthyretin (ATTR), either in its hereditary (ATTRv) or acquired (ATTRwt) form, being the latter mainly age-related.[1,2] Although it was thought to be a rare disease, some authors report a mean prevalence of ATTR-CA of 11% among patients with heart failure and preserved ejection fraction.[3,4] Moreover, the global prevalence in general population is unknown, and probably underestimated.
The diagnosis of CA is usually delayed. A recent study showed an interval of more than 4 years from symptoms onset until the diagnosis is reached in 42% of the patients.[5] This fact has an undoubtedly negative impact in the decline in quality of life and survival of the affected patients. In addition, there are novel pharmacological therapies that are promising for patients with ATTR-CA, decreasing all-cause mortality and cardiovascular-related hospitalizations and exhibiting a slowdown in the capacity and quality of life impairment. Maximal efficacy of the available therapies has been reported for treatment in early stages of the disease.[6,7] For this reason, early diagnosis of ATTR-CA is of the utmost importance.
Prostate cancer is the most frequent neoplasia in male population, excluding cutaneous epithelial tumors. Histological study through transrectal needle biopsy is the cornerstone of prostate cancer diagnosis.[8] As a consequence, a significant number of prostate biopsies are performed and analysed, with a variable percentage of malignancy. Although it is considered a rare finding, a careful pathological analysis of these samples can reveal amyloid deposits.[9] Therefore a prostate biopsy can be the first, and even the only, histological demonstration of amyloidosis in these patients.
There are some previous reports that analysed the relation between PA and cardiac involvement.[10,11] Nevertheless, the definition of CA in these studies is not strict and does not always follow the international diagnostic recommendations. Moreover, information on genetic testing is lacking in most of the reported cases. In our study, based on a cohort of cases diagnosed with PA between 2014 and 2023, we aimed to clarify the role of this finding as a potential early marker of CA, based on strict clinical, imaging and histological criteria, and its potential clinical and therapeutic implications.
A cohort of 19 patients diagnosed with amyloid deposition in prostate specimens between 2014 and 2023 was used for this study. In all patients, the diagnosis of prostatic amyloid deposits was confirmed with Congo Red stain and immunohistochemistry. Patients with a finding of amyloid in the prostate were systematically contacted by a cardiologist, who offered to perform a targeted physical examination, an ECG, an echocardiogram, and a 99mTc-DPD scintigraphy study to complete the screening for CA, as well as a genetic test to complete the study of ATTR. All patients underwent serum free light chain assay, and serum and urine protein electrophoresis with immunofixation.
Patients in which the initial diagnosis of amyloidosis was done in prostate samples were the subject of this study, including both needle biopsies and surgical specimens, all of them studied by one and only pathologist. Formalin-fixed paraffin-embedded sections had been routinely stained with hematoxylin-eosin. To confirm the diagnosis of amyloidosis, Congo Red staining was performed as well as high sensitivity immunohistochemical analysis with monoclonal antibodies against TTR, A amyloid, kappa and lambda light chains, using an enzyme-conjugated multimer complex in an automatic stainer (OptiView DAB Detection Kit®, Benchmark Ultra®, Ventana Medical Systems, Illkirch, France).
Continuous data were presented as median ± interquartile range (IQR) and compared using Mann Whitney U test (alpha level of 0.05 (5%) was used). Categorical data were presented as counts (proportions). All analyses were performed using STATA software, version 15 (Stata Corp, College Station, Texas, USA).
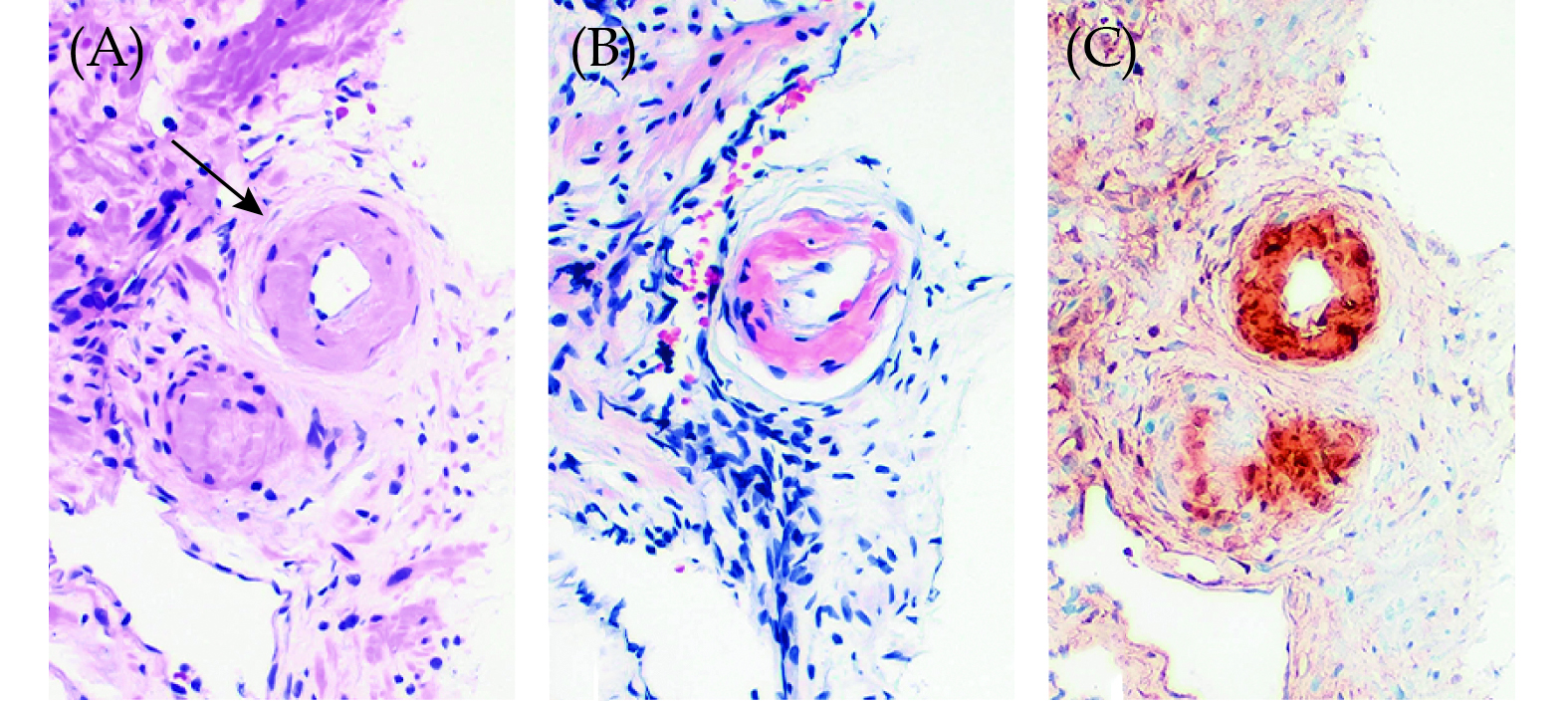
A total of 19 out of 1392 patients were prospectively diagnosed with incidental PA between 2014 and 2023, corresponding to sixteen 12-core transrectal ultrasound-guided needle biopsies, two radical prostatectomy surgical specimens and one transurethral resection sample. Histopathological study revealed amyloid deposition, with variable extension, that presented mainly in the walls of small size vessels, predominantly small arteries, in all the cases except one needle biopsy in which the deposits occurred preferentially in the stroma (Figure 1A and 1B). Immunohistochemical stains were positive for TTR and negative for amyloid A and light chains in 18 of the 19 patients included in the study (Figure 1C). The remaining case, which corresponded to a patient previously diagnosed of multiple myeloma, showed equivocal results in immunohistochemistry, which was unconclusive.
The median age of the patients was 80.2 years (IQR = 74.9–82.6 years), and 13 patients (68.4%) were concomitantly diagnosed with prostate adenocarcinoma. Baseline population characteristics are summarized in Table 1.
| VARIABLES | VALUES | |
| Comorbidities | Age, yrs | 80.2 ± 7.7 |
| Arterial hypertension | 68.4% | |
| Diabetes mellitus | 36.8% | |
| Dyslipidemia | 73.7% | |
| COPD/Asthma | 5.3% | |
| SAS | 0 | |
| Prostate Adenocarcinoma | 68.4% | |
| ISUP Grade 1 | 23.0% | |
| ISUP Grade 2 | 30.8% | |
| ISUP Grade 3 | 15.4% | |
| ISUP Grade 4 | 15.4% | |
| ISUP Grade 5 | 15.4% | |
| Supplementary Tests | Creatinine, mg/dL | 1.01 ± 0.38 |
| LVEF | 55% ± 7% | |
| IVS, mm | 14 ± 4 | |
| Vmax aortic valve, m/s | 1.5 ± 0.4 | |
| E/e´ratio | 10.9 ± 8.2 | |
| TAPSE, mm | 22.7 ± 7.8 | |
| Data are presented as median ± IQR unless other indicated. COPD: chronic obstructive pulmonary disease; GS: Gleason Score; ISUP: International Society of Urological Pathology; LVEF: left ventricle ejection fraction; IVS: interventricular septum; SAS: sleep apnea syndrome; TAPSE: tricuspid annular plane systolic excursion; Vmax: maximal velocity. | ||
Serum free light chain assay, and serum and urine protein electrophoresis with immunofixation was performed for all patients. Clonal plasma cell dyscrasia was only detected in one case (the one with the prior stablished diagnosis of multiple myeloma). Sixteen patients underwent genetic testing (84.21%, 3 patients refused for personal preferences), and no ATTR pathogenic variants were found in any of them.
The median follow-up of the patients since prostate biopsy was 38.0 months (IQR: 16.7–56.4 months). During the follow-up, five patients died. Of them, only one case had been diagnosed with ATTR-CA, and the etiology of death was infectious (7.1 years after the cardiological diagnostic). The other four patients died of heart failure (two of them), infectious disease (the patient with multiple myeloma) and lung cancer. Echocardiography did not show characteristic features of ATTR-CA for none of them. Negative scintingraphy ruled out ATTR-CA in one of them, and it was not performed in remaining three cases (one of them refused and the other two died before having performed it).
All patients underwent an ECG. A vast majority, 17 patients (89.47%), agreed to undergo a cardiological study aimed at screening for myocardial deposits consisting of an echocardiography study. Most of these patients (13 patients) agreed to complete the evaluation with a 99mTc-DPD scintigraphy. The refusal reasons were very advanced age in one case (94 years-old), death before the exam in two cases and personal preferences in another case (significant psychological affectation due to underlying diagnosis -multiple myeloma and prostate adenocarcinoma, respectively). Study flowchart is depicted in Figure 2.
The diagnosis of ATTR-CA was only considered as definitive in patients with PA in whom scintigraphy showed grade 2 or 3 myocardial uptake of radiotracer, following international recommendations.[1] According to this, six patients were eventually diagnosed with ATTR-CA. Considering only those patients with PA who accepted to undergo a full cardiological study, including scintigraphy (13 patients), the prevalence of ATTR-CA rises up to 46,15%.
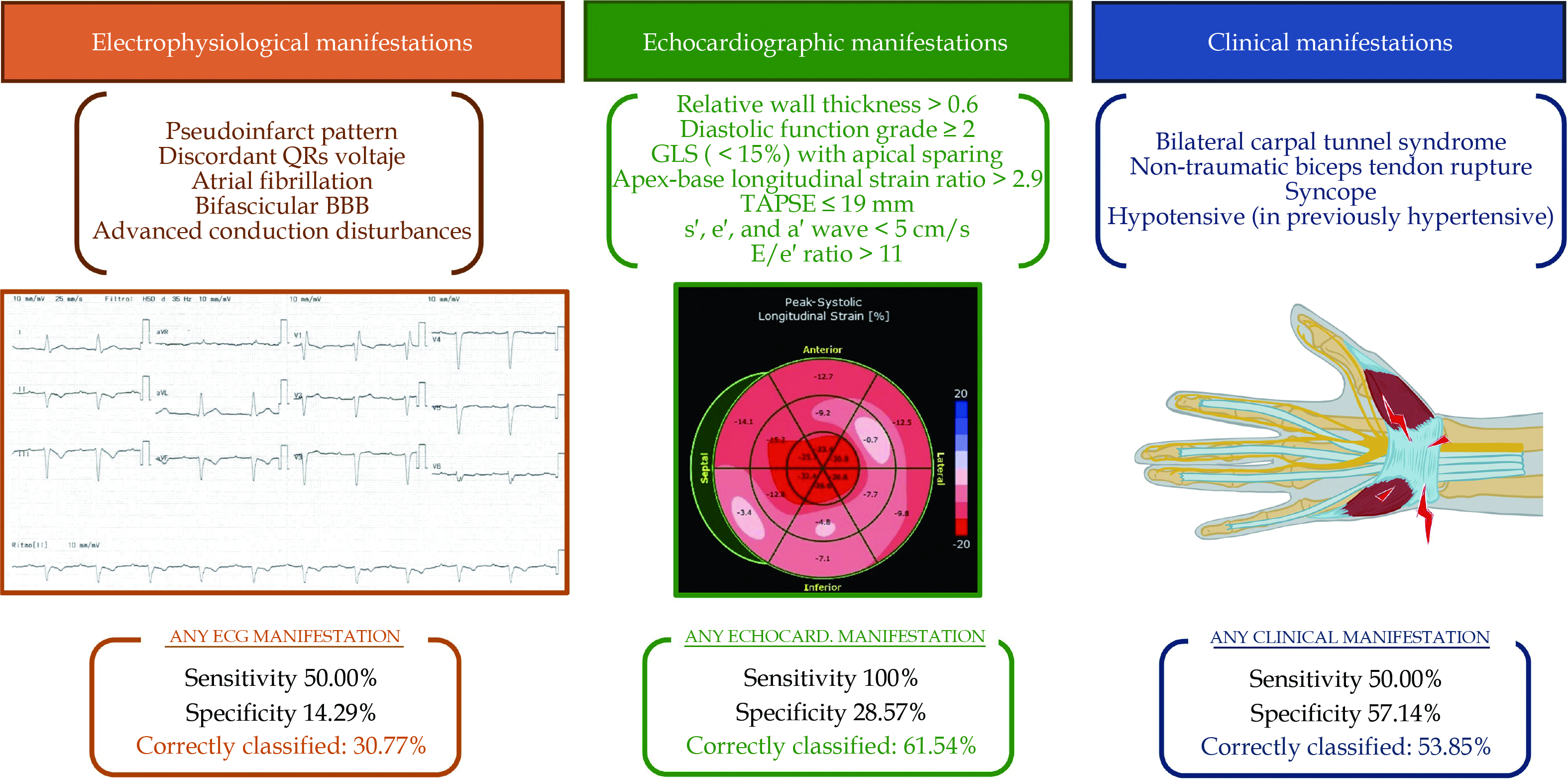
An ECG analysis was performed to search for features typically associated with ATTR-CA. The presence or absence of any of the characteristic hallmarks (pseudoinfarct pattern, discordant QRS voltage regarding ventricle wall thickness, atrial fibrillation, bifascicular bundle branch block or advanced atrio-ventricular block) had a poor sensitivity (50.0%), specificity (14.29%) and overall accuracy (30.77%), as shown in Figure 3.
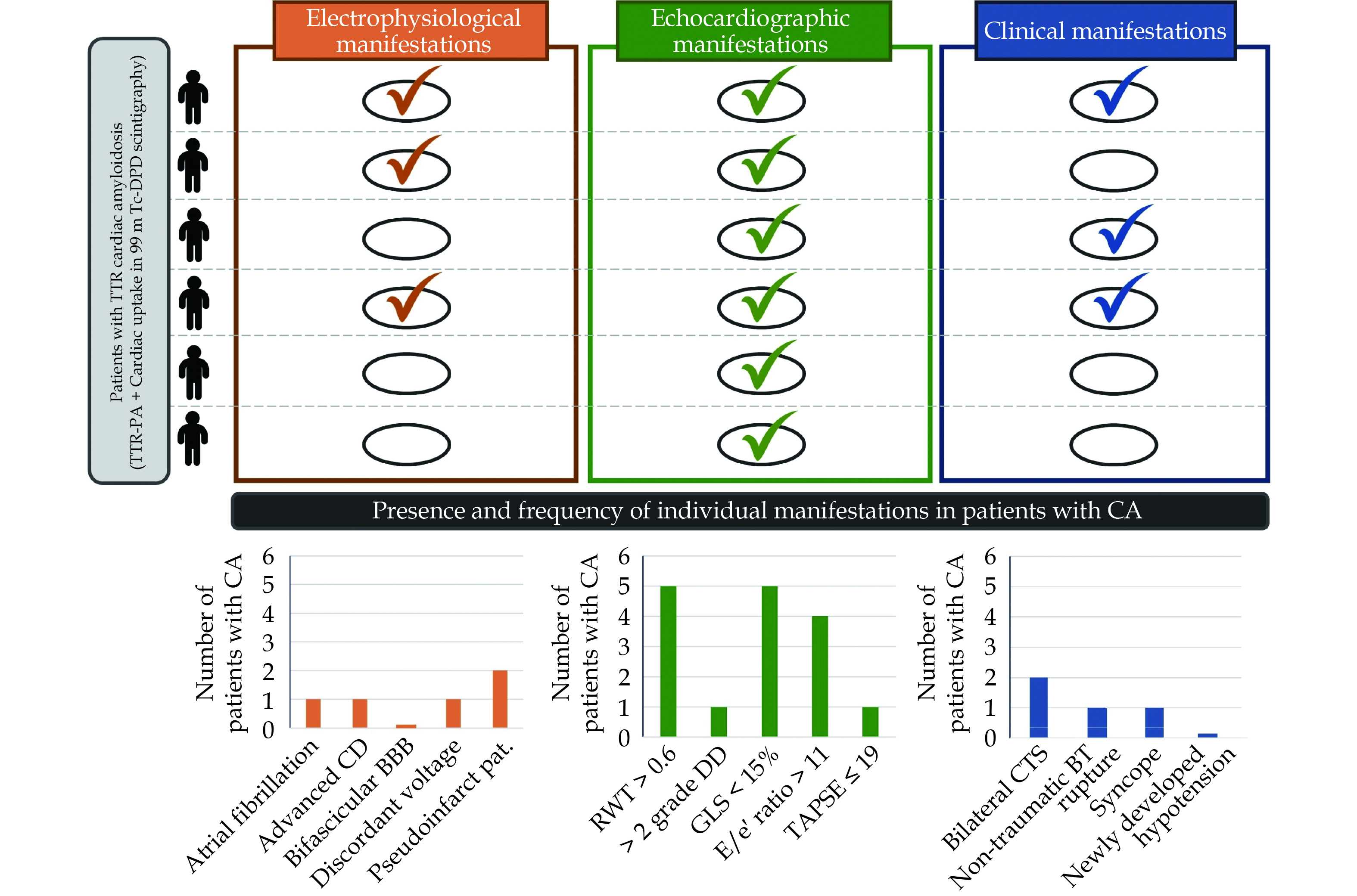
Echocardiography was performed to 17 patients. Echocardiographic criteria stated in consensus documents[12] were assessed for all patients. Only two patients fulfilled the diagnostic score, and both were eventually diagnosed with definitive CA. None of the patients without ATTR-CA fulfilled the echocardiographic diagnosis score. Although sensitivity of the presence of any echocardiographic criteria was high, specificity and accuracy were poor as well (Figure 3). The prevalence of each individual ECG and echocardiographic criteria in CA patients is displayed in Figure 4.
99mTc-DPD scintigraphy was performed in 13 patients. Those with cardiac uptake grade 2 or 3 were therefore diagnosed with CA. Median time from prostate biopsy to scintigraphy performance was 2.3 months (IQR = 1.9–16.2 months). In patients with a positive result, median time to scintigraphy was higher than in non-positive patients, although differences between groups were non-significant, probably due to the small size of the sample (8.89 vs. 2.29 months, P = 0.8301).
A wide clinical study was directed to explore traditional clinical “red-flags”. Only a few clinical characteristics were found. The most frequently detected were musculo-skeletal manifestations, as bilateral carpal tunnel syndrome and non-traumatic rupture of the biceps tendon (Figure 4). Nevertheless, the presence of clinical “red-flags” demonstrated to be an inaccurate criteria for accepting or ruling out the diagnosis of ATTR-CA, as shown in Figure 3.
All the patients eventually diagnosed with ATTR-CA were asymptomatic at the time of PA diagnosis and scintigraphy. One of them developed heart failure in the follow-up (time to symptom onset since scintigraphy was 11.7 months) and another one underwent a pacemaker implantation due to alternating bundle branch block detected 15.8 months after scintigraphy.
The histological detection of amyloid deposition in prostate samples can be the first evidence of amyloidosis in some individuals. To the best of our knowledge, our study is the first to prospectively assess the role of the recognition of amyloid deposits in prostate biopsies as an early marker of cardiac involvement, in accordance with international guidelines diagnostic criteria of ATTR-CA.
ATTR-CA diagnosis was made in those patients in which prostatic ATTR deposits were found and presented with a cardiac uptake equal or higher than grade 2 in 99mTc-DPD scintigraphy. We found that 46.15% of the patients with PA deposits, undergoing an scintigraphy, were also diagnosed with ATTR-CA. All of them were asymptomatic at the time of prostate biopsy analysis and scintigraphy.
Prostate cancer is the most commonly diagnosed cancer in men, excluding non-melanocytic skin neoplasms. Transrectal biopsy is the gold standard diagnostic tool, and is indicated for patients at high risk of prostate cancer, based on calculators that combine clinical and biochemical data.[8,13] Prostate biopsies as well as surgical resection specimens represent a large workload in most Pathology departments. Localized amyloidosis of the seminal tract is not too rare as a histological finding in needle biopsies and, principally in radical prostatectomy specimens.[14] It consists of subepithelial deposits in the seminal vesicles and ejaculatory ducts, without affecting vessel walls. It has been characterized as Semenogelin 1 and it was not associated with subsequent development of systemic amyloidosis.[14,15] It is much more unusual the finding of amyloid deposition in the prostate, that has been only described in some case reports and isolated series, characterized as ATTR by immunohistochemistry or mass spectrometry.[16–18] In opposition to seminal vesicle amyloidosis, a localized disease, prostate deposits of amyloid usually occur in the blood vessel walls (mainly in the muscular wall of small arteries, although small veins deposits have been also reported) suggesting a possible systemic involvement.[9] Genetic study of these patients has not been routinely performed in previous reports. However, three case of ATTRv have been reported to the best of our knowledge, two of them with a Val30Met heterozygous mutation and the other one with THr60A1a mutation.[14,19] It is worth noting that these mutations can also be associated with ATTR-CA.[20,21]
Amyloid deposits in prostate tissue are a rare finding, but given the large number of prostate biopsies performed daily and the possible association of PA with cardiac involvement they should not be considered as an insignificant source of clinical information. These findings can complement or even eliminate the need for biopsies traditionally obtained from other locations (e.g., salivary glands, abdominal fat or skin). In our Healthcare Area, the incidence of PA was 1.36% of all prostate needle biopsies, which is similar to the incidence reported in other series.[11]
A recent position paper of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases on diagnosis and treatment of CA has recognized the role of extracardiac biopsies for CA diagnois: definitive diagnosis can be stablished if the presence extracardiac amyloidosis deposits is accompanied by characteristic echocardiographic and magnetic resonance features, in the absence of an alternative cause for increased left ventricular wall thickness.[12,22]
In our case series, we decided to enhance the cardiovascular study with scintingraphy, given the high specificity for ATTR-CA when the disease is suspected, in the absence of conclusive results of the echocardiographic evaluation.[23] There are some previous studies describing the relationship of PA and heart involvement but diagnostic criteria employed for ATTR-CA were much less strict than ours.
In a recent case series of six patients with ATTR-PA,[10] five individuals were eventually diagnosed with ATTR-CA, detected by endomyocardial biopsy in one case, scintigraphy in another case, and based just on cardiac biomarkers and non-specified echocardiogram findings in the remaining three cases, being the latter diagnostic criteria very vague regarding those gathered in international consensus documents. As a matter of fact, none of the biomarkers are validated to make the final diagnosis and, as we observed in our cohort, classic echocardiographic features may have a significant diagnostic imprecision.
Another recent study that comprised 36 patients with PA reported that 32.5% of the patients presented concomitant cardiovascular symptoms as arrhythmias, conduction defects and/or hypertrophy. Unfortunately, authors did not perform further cardiac studies needed for assessing a ATTR-CA final diagnosis.[11]
Dasari, et al.[24] reported the retrospective evaluation of 672 amyloid specimens in urinary bladder, tendon/sinovium and prostate. They only had clinical information of 62 patients from the initial global cohort, of which 20 developed ATTR-CA (supporting the diagnosis on scintigraphy and cardiac imaging criteria). Only 81 specimens from the initial cohort corresponded with prostate samples (33.3% were ATTR subtype), but there was no information on how many patients were eventually diagnosed with ATTR-CA in PA subgroup. However, the authors report very interesting data on the high amyloid type concordance between the extracardiac and cardiac histological findings in five patients where they were both available. This fact reinforces the value of PA finding as a good marker for an early diagnosis and typification of cardiac involvement.
Based on our results, the diagnosis of PA should be followed by a specific and complete cardiological examination, as almost half of the patients can be diagnosed with CA. A simple approach, based on clinical, EGC and echocardiographic markers may not be accurate enough for assessing cardiac involvement in this population, being the scintigraphy needed for a final ATTR-CA diagnosis.
A very important finding in our study has been that most of the patients that were diagnosed with ATTR-CA were free of cardiovascular symptoms. This is a very relevant point, given the significant CA diagnostic delay that usually occurs. Early diagnosis is of utmost importance, as recently approved therapies for CA have shown significantly better results specifically in early stages of disease.[7] Although the added value of disease-modifying treatment is debatable in an elderly population frequently concomitantly diagnosed with adenocarcinoma, an early diagnosis in this group of patients also allows for the development of preventive strategies for common cardiovascular complications often observed in patients with CA. These complications are especially relevant in this age range and include orthostatic syncope or bradyarrhythmias. For example, cautious use of antihypertensive therapies or beta-blockers in these patients can be considered.
As recognition of PA depends largely on the pathologist’s awareness (as amyloid deposits can be isolated and subtle),[11] emphasis must be placed on the significance of screening for this finding, alongside the primary objective of the biopsy: the diagnosis of cancer. Routine employment of the Congo Red stain could be considered, given the important potential impact. Finally, encouraging multidisciplinary collaboration among cardiologists, pathologists and urologists is essential, given the significant potential impact on the early diagnosis of CA.
In this article, we analyzed the role of the amyloid deposits in prostate as a marker of concomitant early stage ATTR-CA, through a systematic cardiological examination, in accordance with international guidelines diagnostic criteria.
Given the low prevalence of amyloid deposits in prostate, the cohort sample size is small, probably limiting the statistical analysis. Nevertheless, the systematic nature of the clinical evaluation allowed us to deepen the cardiology study of patients, reaching a more precise evaluation of the clinical implication of histological findings.
Additionally, as the study was based on prostate biopsies and specimens obtained as part of oncological screening, younger populations are underrepresented. Further studies with a larger sample size should be conducted for evaluating the clinical value of PA findings in this population.Besides, we found that, although non-significant, time from biopsy to scintigraphy was longer in patients that were finally diagnosed with CA. This could raise the question on whether PA is a very early marker of systemic amyloidosis and high clinical suspicion should be kept, especially if symptoms are developed, as cardiac involvement may eventually occur over the years. Probably, longer follow-up periods will be necessary for further conclusions.
Finally, mass spectometry was not available to complete the study of the samples, as it usually occurs in a real word setting. However, the immunohistochemistry techniques used in our study, based on the use of conjugated multimers, offer a high sensitivity and specificity, assured by the use of positive and negative controls.
None.
Conceptualization: M.C.F, E.E.C, J.S.R, S.A.S; S.R.R, C.T.B; E.A.A; S.T; P.D.A; JA.O.R; Methodology: M.C.F; E.E.C; J.S.R; S.R.R; E.C.O; JA.O.R; Investigation: M.C.F; E.E.C; R.C.P; JA.O.R; Formal analysis: M.C.F; E.E.C; R.C.P; S.R.R.; JA.O.R; Writing – original draft: M.C.F; E.E.C; R.C.P; E.C.O; JA.O.R; Writing – review & editing: M.C.F; E.E.C; J.S.R; S.A.S; S.R.R; C.T.B; R.C.P; S.T; P.D.A; E.A.A; P.G.P; ME.E.C; E.C.O; JA.O.R; All authors read and approved the final manuscript. We want to thank Pablo Mato-Collazo, PhD, for his help in language revision and editing.
| [1] |
Garcia-Pavia P, Rapezzi C, Adler Y, et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur J Heart Fail 2021; 23: 512−526.
|
| [2] |
Bukhari S. Cardiac amyloidosis: state-of-the-art review. J Geriatr Cardiol 2023; 20: 361−375. doi: 10.26599/1671-5411.2023.05.006
|
| [3] |
Magdi M, Mostafa MR, Abusnina W, et al. A systematic review and meta-analysis of the prevalence of transthyretin amyloidosis in heart failure with preserved ejection fraction. American journal of cardiovascular disease 2022; 12: 102−111.
|
| [4] |
Ruberg FL, Grogan M, Hanna M, et al. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol 2019; 73: 2872−2891.
|
| [5] |
Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation 2019; 140: 16−26. doi: 10.1161/CIRCULATIONAHA.118.038169
|
| [6] |
Elliott P, Gundapaneni B, Sultan MB, et al. Improved long-term survival with tafamidis treatment in patients with transthyretin amyloid cardiomyopathy and severe heart failure symptoms. Eur J Heart Fail 2023; 25: 2060−2064. doi: 10.1002/ejhf.2974
|
| [7] |
Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018; 379: 1007−1016. doi: 10.1056/NEJMoa1805689
|
| [8] |
Streicher J, Meyerson BL, Karivedu V, Sidana A. A review of optimal prostate biopsy: indications and techniques. Ther Adv Urol 2019; 11: 1756287219870074.
|
| [9] |
Sibley RR, Ellison B, Siegert JJ, Van Gorder MA. Isolated prostate amyloidosis; a case report. Urol Case Rep 2022; 45: 102229. doi: 10.1016/j.eucr.2022.102229
|
| [10] |
Nevo A, Muchtar E, Stern KL, et al. The Clinical Implication of Incidental Prostatic Amyloidosis. Urology 2020; 145: 253−257. doi: 10.1016/j.urology.2020.08.053
|
| [11] |
Foix MP, Calvo DF, Condom IME, et al. Clinical relevance of amyloid in prostate samples: a report on 40 patients. Histopathology 2022; 81: 363−370. doi: 10.1111/his.14717
|
| [12] |
Garcia-Pavia P, Rapezzi C, Adler Y, et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2021; 42: 1554−1568. doi: 10.1093/eurheartj/ehab072
|
| [13] |
Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2021; 79: 243−262.
|
| [14] |
Nemov I, Hougen HY, Iakymenko OA, Jorda M, Gonzalgo ML, Kryvenko ON. Localized amyloidosis of the seminal tract is not associated with subsequent development of systemic amyloidosis. Urology 2022; 164: 46−49. doi: 10.1016/j.urology.2021.12.008
|
| [15] |
Buxbaum JN, Dispenzieri A, Eisenberg DS, et al. Amyloid nomenclature 2022: update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 2022; 29: 213−219. doi: 10.1080/13506129.2022.2147636
|
| [16] |
Lawrentschuk N, Pan D, Stillwell R, Bolton DM. Implications of amyloidosis on prostatic biopsy. Int J Urol 2004; 11: 925−927. doi: 10.1111/j.1442-2042.2004.00921.x
|
| [17] |
Singh SK, Wadhwa P, Nada R, et al. Localized primary amyloidosis of the prostate, bladder and ureters. Int Urol Nephrol 2005; 37: 495−497. doi: 10.1007/s11255-005-2088-x
|
| [18] |
Wilson SK, Buchanan RD, Stone WJ, Rhamy RK. Amyloid deposition in the prostate. J Urol 1973; 110: 322−323. doi: 10.1016/S0022-5347(17)60202-7
|
| [19] |
Maeda K, Kubota Y, Kitagawa S, Ueda M, Ando Y, Ito Y. The prostate as a good candidate organ for proving amyloid deposition in non-endemic late onset hereditary transthyretin amyloidosis: Report of two cases. J Neurol Sci 2021; 424: 117418. doi: 10.1016/j.jns.2021.117418
|
| [20] |
Koike H, Nakamura T, Nishi R, et al. Cardiac and peripheral vasomotor autonomic functions in hereditary transthyretin amyloidosis with non-Val30Met mutation. Amyloid 2019; 26: 13−14. doi: 10.1080/13506129.2019.1582023
|
| [21] |
Ripoll-Vera T, Buades J, Cisneros E, et al. Cardiac Involvement in a Patient Cohort With Val30Met Mutation Transthyretin Amyloidosis. Revista espanola de cardiologia (English ed) 2019; 72: 92−94. doi: 10.1016/j.recesp.2017.09.006
|
| [22] |
Kittleson MM, Ruberg FL, Ambardekar AV, et al. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2023; 81: 1076−1126.
|
| [23] |
Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016; 133: 2404−2012. doi: 10.1161/CIRCULATIONAHA.116.021612
|
| [24] |
Dasari S, Dispenzieri A, Mansour S, et al. Non-cardiac biopsy sites with high frequency of transthyretin amyloidosis. ESC Heart Fail 2021; 8: 750−755. doi: 10.1002/ehf2.13130
|
| [1] | Laura Samaniego-Vega, Ana Ayesta-Lopez, Elena Valle-Calonge, Jesus M. De La Hera-Galarza, Jose Gutierrez-Rodriguez, Pablo Solla-Suarez. In the early diagnosis of cardiac amyloidosis by point-of-care ultrasound (POCUS) in older patients with heart failure: towards a new standard of care?[J]. Journal of Geriatric Cardiology, 2024, 21(12): 1147-1148. DOI: 10.26599/1671-5411.2024.12.003 |
| [2] | Syed Bukhari. Cardiac amyloidosis: state-of-the-art review[J]. Journal of Geriatric Cardiology, 2023, 20(5): 361-375. DOI: 10.26599/1671-5411.2023.05.006 |
| [3] | Koji Takahashi, Mina Yamashita, Tomoki Sakaue, Daijiro Enomoto, Shigeki Uemura, Takafumi Okura, Shuntaro Ikeda, Takanori Senba, Akira Saijo, Nobuhisa Yamamura, Sohei Kitazawa. Light chain cardiac amyloidosis in a nonagenarian[J]. Journal of Geriatric Cardiology, 2022, 19(1): 83-89. DOI: 10.11909/j.issn.1671-5411.2022.01.008 |
| [4] | Jing LI, Hong-Yan WANG, Ning BIAN, Ru-Yi XU, Can HUA, Shao-Li NIU, Zhuo-Kun GAN, Qing WANG, Hai-Tao TIAN. Cardiac involvement in light chain amyloidosis: a case report[J]. Journal of Geriatric Cardiology, 2020, 17(6): 373-378. DOI: 10.11909/j.issn.1671-5411.2020.06.007 |
| [5] | Christopher Strouse, Alexandros Briasoulis, Rafael Fonseca, Yogesh Jethava. Approach to a patient with cardiac amyloidosis[J]. Journal of Geriatric Cardiology, 2019, 16(7): 567-574. DOI: 10.11909/j.issn.1671-5411.2019.07.010 |
| [6] | Eiji Ichimoto, Adam Arnofsky, Michael Wilderman, Richard Goldweit, Joseph De Gregorio. Early mortality and safety after transcatheter aortic valve replacement using the SAPIEN 3 in nonagenarians[J]. Journal of Geriatric Cardiology, 2018, 15(6): 387-393. DOI: 10.11909/j.issn.1671-5411.2018.06.002 |
| [7] | Ting–Zhi DENG, Bai–Qing OU, Dao–Quan PENG. The eyes are the window to the heart: one case of cardiac amyloidosis with eyelid swelling as the initial symptom[J]. Journal of Geriatric Cardiology, 2017, 14(11): 712-714. DOI: 10.11909/j.issn.1671-5411.2017.11.008 |
| [8] | Marc-Alexander Ohlow, Ting-Hui Chen, Andreas Schmidt, Joerg Saenger, Bernward Lauer. Clinical profile of patients with advanced age and inflammatoric dilated cardiomyopathy on endomyocardial biopsy[J]. Journal of Geriatric Cardiology, 2015, 12(6): 605-612. DOI: 10.11909/j.issn.1671-5411.2015.06.007 |
| [9] | Jong Shin Woo, Tae-Kyung Yu, Woo-Shik Kim, Kwon Sam Kim, Weon Kim. Early prediction of myocardial viability after acute myocardial infarction by two-dimensional speckle tracking imaging[J]. Journal of Geriatric Cardiology, 2015, 12(5): 474-481. DOI: 10.11909/j.issn.1671-5411.2015.05.002 |
| [10] | Geng QIAN, Chen WU, Yang ZHANG, Yun-Dai CHEN, Wei DONG, Yi-Hong REN. Prognostic value of high-sensitivity cardiac troponin T in patients with en-domyocardial-biopsy proven cardiac amyloidosis[J]. Journal of Geriatric Cardiology, 2014, 11(2): 136-140. DOI: 10.3969/j.issn.1671-5411.2014.02.011 |
| VARIABLES | VALUES | |
| Comorbidities | Age, yrs | 80.2 ± 7.7 |
| Arterial hypertension | 68.4% | |
| Diabetes mellitus | 36.8% | |
| Dyslipidemia | 73.7% | |
| COPD/Asthma | 5.3% | |
| SAS | 0 | |
| Prostate Adenocarcinoma | 68.4% | |
| ISUP Grade 1 | 23.0% | |
| ISUP Grade 2 | 30.8% | |
| ISUP Grade 3 | 15.4% | |
| ISUP Grade 4 | 15.4% | |
| ISUP Grade 5 | 15.4% | |
| Supplementary Tests | Creatinine, mg/dL | 1.01 ± 0.38 |
| LVEF | 55% ± 7% | |
| IVS, mm | 14 ± 4 | |
| Vmax aortic valve, m/s | 1.5 ± 0.4 | |
| E/e´ratio | 10.9 ± 8.2 | |
| TAPSE, mm | 22.7 ± 7.8 | |
| Data are presented as median ± IQR unless other indicated. COPD: chronic obstructive pulmonary disease; GS: Gleason Score; ISUP: International Society of Urological Pathology; LVEF: left ventricle ejection fraction; IVS: interventricular septum; SAS: sleep apnea syndrome; TAPSE: tricuspid annular plane systolic excursion; Vmax: maximal velocity. | ||