| Citation: | Please cite this article as: HU SS, the Writing Committee of the Report on Cardiovascular Health and Diseases in China. Epidemiology and current management of cardiovascular disease in China. J Geriatr Cardiol 2024; 21(4): 387−406. DOI: 10.26599/1671-5411.2024.04.001. |
The Annual Report on Cardiovascular Health and Diseases in China (2022) intricate landscape of cardiovascular health in China. This is the fourth section of the report with a specific focus on epidemiology and current management of cardiovascular disease (CVD) in China. This section of the report highlights the epidemiological trends of CVD in China. It reveal a concerning rise in prevalence, with approximately 330 million affected individuals, including significant numbers with stroke, coronary artery disease (CAD), heart failure, and other conditions. CVD stands as the primary cause of mortality among both urban and rural populations, accounting for nearly half of all deaths in 2020. Mortality rates are notably higher in rural areas compared to urban centers since 2009. While age-standardized mortality rates have decreased, the absolute number of CVD deaths has increased, primarily due to population aging. Ischemic heart disease, hemorrhagic and ischemic strokes are the leading causes of CVD-related deaths. Notably, the burden of atherosclerotic cardiovascular disease has risen substantially, with atherosclerotic cardiovascular disease-related deaths increasing from 1990 to 2016. The incidence of ischemic stroke and ischemic heart disease has shown similar increasing trends over the past three decades. CAD mortality, particularly acute myocardial infarction, has been on the rise, with higher mortality rates observed in rural areas since 2016. The prevalence of CAD has increased significantly, with over 11 million patients identified in 2013. Studies assessing hospital performance in managing acute coronary syndrome reveal gaps in adherence to guideline-recommended strategies, with disparities in care quality across hospitals. However, initiatives like the China Patient-centered Evaluative Assessment of Cardiac Events (PEACE)-Retrospective AMI Study and the Improving Care for Cardiovascular Disease in China-Acute Coronary Syndrome (CCC-ACS) project aim to improve patient outcomes through enhanced care protocols. Moreover, advancements in medical technology, such as quantitative flow ratio-guided lesion selection during percutaneous coronary intervention, show promise in improving clinical outcomes for patients undergoing intervention.
The prevalence of CVD in China is on the rise. The number of patients with CVD is estimated to be 330 million, including 13 million with stroke, 11.39 million with coronary artery disease (CAD), 8.9 million with heart failure (HF), 5 million with pulmonary heart disease, 4.87 million with atrial fibrillation, 2.5 million with rheumatic heart disease, 2 million with congenital heart disease, 45.3 million with peripheral artery disease and 245 million with hypertension.[1]
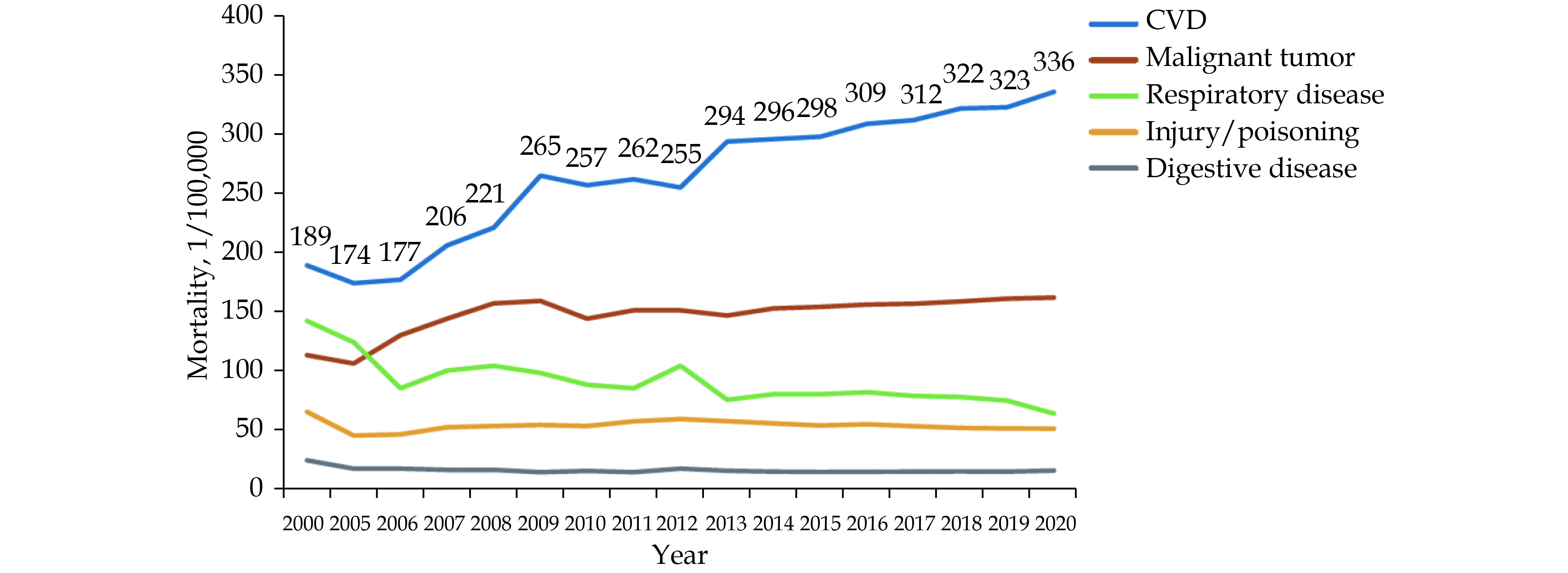
In 2020, the mortality of CVD still ranked first, higher than that of tumor and other diseases (Figures 1 & 2). The mortality of CVD in rural areas has exceeded and continued to be higher than the urban level since 2009 (Figure 3).
In 2020, the mortality of CVD in rural areas was 336.13/100,000, of which, 171.36/100,000 for heart diseases and 164.77/100,000 for cerebrovascular diseases, while the mortality of CVD in urban areas was 291.04/100,000, of which, 155.86/100,000 for heart diseases and 135.18/100,000 for cerebrovascular diseases.
CVD is the leading cause of death among urban and rural residents in China. It accounted for 48.00% and 45.86% of all deaths in rural and urban areas in 2020, respectively (Figures 4 & 5). Two out of five deaths were attributable to CVD.
The National Mortality Surveillance System covers 300 million people from 605 surveillance points in 31 provincial administrative units of China, accounting for 24% of China’s population. This system collects detailed information on population mortality through an internet-based approach, estimates the standardized rate based on the 2010 census data, and calculates the burden of CVD premature mortality using the year of life lost (YLL) as the main parameter.[2] The study revealed that the estimated CVD deaths increased from 3.09 million in 2005 to 4.58 million in 2020; the age-standardized mortality rate (ASMR) decreased from 286.85 per 100,000 in 2005 to 245.39 per 100,000 in 2020, males had higher ASMR than females in each survey year (Figure 6). The age-standardized YLL rate of total CVD fell by 19.27% between 2005 and 2020. Though CVD premature mortality burden decreased in China, but still higher than global average, there was a 48.06% increase in total CVD deaths between 2005 and 2020. Population ageing was dominant driver contributed to CVD deaths increase, followed by population growth.
In 2020, ischemic heart disease (IHD), hemorrhagic stroke and ischemic stroke were the three main causes of CVD death in China, IHD occupied 50%–60% on average of CVD premature mortality burden among those who aged 15–50 years. Marked differences were observed in geographical patterns for total CVD and its subcategories, and it appeared to be lower in areas with higher economic development. In 2020, the top 3 provincial administrative units with highest ASMR were Tibet (416.07 per 100,000), Heilongjiang (346.56 per 100,000), Henan (331.38 per 100,000), and the last 3 were Shanghai (137.10 per 100,000), Zhejiang (144.40 per 100,000) and Jiangsu (148.11 per 100,000). Despite in the condition of general reduction for CVD mortality in China, some provincial administrative units, such as Anhui, Shanxi and Tibet experienced an upward trend of ASMR for total CVD.
One important feature of the epidemiology of CVD in China is that the burden of ASCVD has rapidly and substantially increased.[3] There were approximately 2.4 million deaths from ASCVD in 2016, representing 61% of deaths from CVD and 25% of all deaths, an increase from around 1 million deaths from ASCVD (40% of deaths from CVD and 11% of all deaths) in 1990. The rise in ASCVD mortality from 1990 to 2016 was due to remarkably increased rates of death from IHD and slightly increased rates of death from ischemic stroke.[4] However, the mortality data do not adequately describe the magnitude of the two subtypes of ASCVD, because ischemic stroke has the much lower mortality to incidence ratio than that of IHD. The incidence rates and increasing trends of ischemic stroke and IHD were nearly identical over the past 3 decades.
In the 1980s and early 1990s, IHD incidence rates and mortality were very low among the Chinese population.[5] The trend for an increasing burden of IHD in China began in the 1980s and has become more marked in the past 20 years. IHD was the cause of approximately 1.7 million deaths in China in 2016 and was the second leading cause of death in the Chinese population,[4] but IHD was ranked as the seventh leading cause of premature death in 1990. Ischemic stroke was the cause of approximately 0.73 million deaths in China in 2016, accounting for nearly 40% of deaths from stroke. The incidence rate of ischemic stroke in China is 36% higher than the global average (240.58 per 100,000 person-years vs. 176.44 per 100,000 person-years), but with a lower mortality to incidence ratio (0.19 vs. 0.24).[6]
Over the past 10 years in China, approximately 1.5–1.7 million new-onset hemorrhage strokes have occurred annually. Hemorrhagic stroke accounted for around 30% of new-onset strokes, but 60% of deaths from stroke. Both crude mortality and age-standardized mortality from hemorrhagic stroke have declined considerably in recent decades, and the proportion of all CVD deaths that were caused by hemorrhagic stroke dropped from 39% in 1990 to 27% in 2016.[4] Even with this remarkable decline in mortality from hemorrhagic stroke, China remained one of the countries with the highest burdens of hemorrhagic stroke in a comparison of 110 countries. The incidence and mortality of hemorrhagic stroke in China were nearly twice the global average. The decline in mortality might be largely due to reduced case fatality as a result of improved medical treatment, because the incidence rates of hemorrhagic stroke in China have remained stable or have even slightly increased according to available data from the Global Burden of Disease study.
Marked regional differences have been observed in epidemiological trends in ASMR from IHD. In 2015, the mortality from IHD in Heilongjiang in the Northeast region and Shanghai in the Southeast region was 187.4 per 100,000 and 44.2 per 100,000, respectively, a 4.2-fold difference between the regions.[7] Between 1990 and 2015, 22 out of 33 provincial administrative units in China had increasing ASMR from IHD, and the increase was > 30% in 8 of the provincial administrative units. The general population in Qinghai had a 54% increase in mortality from IHD (ranked first) and a 278.8% increase in the number of deaths from IHD.
Large variations in ASMR and incidence of stroke between regions also existed. The male population of Guizhou and the female population of Tibet have 3.5–4.0 fold higher mortality from stroke than the male and female populations of Hong Kong. The general population in Northeast China has a 2.4-fold higher incidence and a 1.4-fold higher mortality from stroke than populations in the South of China.[8]
Over the past 30 years, the general population in China has experienced dramatic improvements in health status, as indicated by substantially increased life expectancy and healthy life expectancy. The improvements have led to a rapid and consistent increase in elderly population, which had a large effect on the burden of CVD and major cardiovascular risk factors. One study with the use of a Markov computer simulation model found that with population growth and ageing, the projected annual CVD events would increase by > 50% between 2010 and 2030, and current risk factor trends would add another 23% increase in CVD events during this period.[9] Another study indicated that the number of nonfatal or fatal CAD events would increase during this period, mostly in people aged 65–84 years.[10]
Ageing patients with CVD present many challenges beyond merely a large number of elderly patients. Firstly, current randomized controlled trials lack evidence of primary and secondary prevention and emergency treatment strategies for CVD in elderly individuals aged ≥ 75 years. Secondly, most elderly patients with CVD coexist with multiple diseases, and there is almost no clear recommendation in the guidelines for this. The decrease in age-specific mortality of CVD plays an important role in reducing CVD mortality. The mortality of younger individuals has significantly decreased, but the elderly still maintains a high level and even increase.
Due to the improvement of medical conditions and health environment, as well as the advancement of medical technology, more CVD patients have received timely diagnosis and treatment, as well as correct care after discharge. The ASMR and age-standardized YLL rate of CVD in China are both showing a downward trend. However, it cannot be ignored that the aging population and increasing population in China have led to an increase in CVD crude mortality and absolute increase in the number of CVD deaths, especially the accelerating trend of CVD mortality in rural areas. This poses challenges to China’s disease prevention and control strategies and the provision of healthcare resources. It is necessary to conduct more researches among the elderly population to further understand the impact of aging on the burden of CVD in China, in order to develop effective strategies to deal with these challenges.
According to the 5th National Health Services Survey in 2013,[11] the prevalence of CAD was 10.2‰ among residents aged ≥ 15 years, 12.3‰ in urban areas, 8.1‰ in rural areas. The prevalence was 27.8‰ for those aged ≥ 60 years. Compared with the data of the fourth survey in 2008 (urban: 15.9‰, rural: 4.8‰, and total: 7.7‰), the prevalence in urban areas decreased, while increased in rural and the total prevalence rates. The number of people aged ≥ 15 years in China with CAD was 11,396,104, an increase of about 1.08 million over the number of patients with CAD across all ages in the fourth National Health Services Survey in 2008.
According to “China Health Statistics Yearbook 2021”,[12] the mortality of CAD among urban residents in China in 2020 was 126.91/100,000, and that in rural areas was 135.88/100,000. In both urban and rural areas, the mortality of CAD in males is higher than that in females (Figure 7).
In 2020, the mortality of CAD continued its upward trend since 2012, with a significant increase in rural areas and exceeding the urban level by 2016 (Figure 8).
The overall mortality of acute myocardial infarction (AMI) showed an upward trend from 2002 to 2020. Since 2005, the mortality of AMI had shown a rapid upward trend. The mortality of AMI in rural areas not only exceeded that of urban areas in 2007, 2009, 2010, and 2011, but also significantly increased in rural areas in 2012 and continued to be higher than the urban level since 2013 (Figure 9).
The mortality of AMI increases with age and begins to significantly increase after the age of 40, with an increasing trend similar to an exponential relationship. The above phenomenon can be found in data from 2002 to 2020 for both urban and rural areas, as well as for men and women.
The China Patient-centered Evaluative Assessment of Cardiac Events (PEACE)-Retrospective AMI Study randomly selected 162 secondary and tertiary hospitals from 31 provincial administrative units in China and analyzed 13,815 medical records of patients admitted for ST-segment elevation myocardial infarction (STEMI).[13] Between 2001 and 2011, the estimated national rate of hospital admission for STEMI per 100,000 population increased every year in China, from 3.7 in 2001, to 8.1 in 2006, to 15.8 in 2011 (Figure 10).
Beijing CAD surveillance data showed that the AMI subtypes in hospitalized patients have changed significantly.[14] The age-standardized hospitalization rate for STEMI decreased slightly from 2007 to 2012, while that of non-STEMI (NSTEMI) increased by 3-fold. The ratio of STEMI to NSTEMI decreased dramatically from 6.5:1.0 to 1.3:1.0 (Figure 11).
The Chinese Acute Myocardial Infarction (CAMI) registry study compared the in-hospital outcomes among 1314 patients with AMI from Beijing Collaborative Group data in 1972 and 1973, and 2200 patients in Beijing from China AMI registry in 2013 and 2014.[15] The results showed that, compared with 40 years ago, the mortality of AMI patients in Beijing reduced significantly (1970s vs. 2010s: 24% vs. 2.6%, P < 0.05). From 2013 to 2014, the in-hospital mortality of AMI patients in different levels of hospitals varied significantly, with provincial, municipal, and county-level hospitals being 3.1%, 5.3%, and 10.2%, respectively (Ptrend < 0.001).[16]
In 2013–2016, 29,581 consecutive STEMI patients were enrolled from 80 hospitals with emergency percutaenous coronary intervention (PCI) treatment capacities and with more than 50 STEMI patients registered in CAMI registry study.[17] The study revealed that the in-hospital mortality was 6.3%. The hospital-specific opportunity-based composite score (OBCS) was calculated in combination with the Chinese guidelines and the American quality control criteria for myocardial infarction. The in-hospital mortality rates in hospitals with low OBCS (< 71.1%), median OBCS (71.1%–76.5%) and high OBCS (> 76.5%) were 7.2%, 6.6% and 5.4%, respectively.
CAMI registry study showed that the in-hospital mortality in patients with AMI varied significantly with different body mass index (BMI) level.[18] 35,964 patients diagnosed with AMI were categorized into 4 groups according to BMI level based on WHO criterion: underweight, normal, overweight, and obese groups, respectively. All subgroups showed a trend toward lower in-hospital mortality risk as BMI increased. The in-hospital mortality rates were 11.3%, 6.0%, 4.2% and 3.0%, respectively for underweight, normal, overweight, and obese people.
A cross-sectional study of the impact of COVID-19 pandemic on the acute coronary syndrome (ACS) admissions in Beijing included 38,999 ACS patients.[19] Study period was defined as December 1, 2019 to June 30, 2020, and control period was defined as December 1, 2018 to June 30, 2019. The study revealed that ACS admissions remained low compared to the same time period in previous year. Admissions for STEMI, NSTEMI, and unstable angina reduced by 38.0%, 41.0%, and 63.3% in study period. PCI performed within 24 h were significantly more frequent during study period in patients with STEMI (37.9% vs. 31.7%, P < 0.0001), but significantly less frequent in patients with NSTEMI (7.9% vs. 9.5%, P = 0.049), and in patients with unstable angina (1.7% vs. 3.5%, P < 0.0001). In-hospital mortality rates in patients with ACS were similar during the study period and the control period. It suggested that during COVID-19 pandemic, ACS admissions reduced significantly in Beijing, but the short-term prognosis for ACS was not deteriorated by the pandemic.
The multi-center retrospective cohort study enrolled 2067 COVID-19 patients admitted from 22 tertiary hospitals in China between January 3, 2020 and April 2, 2020 to explore the association between coronary artery calcification and in-hospital mortality and adverse clinical outcomes.[20] The study showed that patients with high coronary artery calcification were at a higher risk of in-hospital death (HR = 1.731, 95% CI: 1.010–2.971, P = 0.046) and adverse clinical outcomes (HR = 1.611, 95% CI: 1.087–2.387, P = 0.018), which highlighted the importance of calcium load testing for hospitalized COVID-19 patients.
Quantitative flow ratio (QFR) FAVOR III is a multi-center, blinded, randomized, sham-controlled trial in China. The study results demonstrated that the 1-year cumulative primary endpoints were much lower in the QFR-guided group compared with the standard angiography-guided group (5.8% vs. 8.8%; difference: −3.0%; 95% CI: −4.7 to −1.4), HR = 0.65 (95% CI: 0.51–0.83, P = 0.0004), driven by fewer myocardial infarctions and ischemia-driven revascularizations in the QFR-guided group.[21] QFR-guided strategy of lesion selection improved 1-year clinical outcomes compared with standard angiography guidance.
QFR was retrospectively analyzed from the angiograms of 1391 patients enrolled in the PANDA III trial. Patients in whom all functionally ischemic vessels (baseline QFR ≤ 0.80) were treated and in whom all non-ischemic vessels (baseline QFR > 0.80) were deferred were termed as having had QFR-consistent treatment; otherwise, they were termed as having had QFR-inconsistent treatment.[22] Patients with QFR-consistent versus those with QFR-inconsistent treatment had the lower risk of two-year major adverse cardiac events (MACE) (8.8% vs. 13.6%, adjusted HR = 0.64, 95% CI: 0.44–0.93), due mainly to reduced ischemia-driven revascularization (2.9% vs. 8.0%, adjusted HR = 0.35, 95% CI: 0.20–0.60), implying that the PCI treatment in accordance with the QFR measurement was associated with improved two-year clinical outcomes.
Computed tomography-derived fractional flow reserve (CT-FFR) A single-center, retrospective study among 243 patients with symptomatic CAD identified by coronary computed tomography angiography (CTA) showed that 72% of invasive coronary angiography could be avoided by using a deep learning algorithm to calculate CTA-based fractional flow reserve (DL-FFRCT) value > 0.8 as a cut-off for intervention. The study suggested that DL-FFRCT could reduce the need for diagnostic coronary angiography when identifying patients suitable for coronary intervention.[23]
Dynamic CT myocardial perfusion imaging and coronary CTA data obtained from 498 symptomatic patients were retrospectively reviewed.[24] ΔCT-FFRsystolic (defined as the difference in CT-FFR values between the proximal and distal ends of the myocardial bridging calculated in the best systolic phase) was higher in patients with versus without myocardial bridging-related myocardial ischemia [median (IQR): 0.12 (0.08–0.17) vs. 0.04 (0.01–0.07), P < 0.001)]. ΔCT-FFRsystolic had the highest sensitivity (91.7%) and negative predictive value (97.8%), which suggested that ΔCT-FFRsystolic could reliably predict the occurrence of myocardial bridging-related ischemia.
A total of 101 patients with documented ACS events and received more than once coronary CTA were recruited.[25] The culprit lesions exhibited significant increase of luminal stenosis, remodeling index, and necrotic core, while decrease of CT-FFR and calcium ratio during follow-up in comparison to that of non-culprit lesion. The XGBoost model comprising the top five important plaque features revealed higher predictive ability [area under the curve (AUC) = 0.918, 95% CI: 0.861–0.968]. Dynamic changes of plaque features were highly relative with subsequent ACS events. The machine learning model of integrating these lesion characteristics improved the ability for predicting risks of ACS events.
Optical coherence tomography (OCT) One study investigated the association of high-risk culprit plaque features by OCT in patients with STEMI.[26] A total of 274 patients were divided into 3 groups based on residual SYNTAX score (rSS): rSS = 0 (n = 72), 0 < rSS ≤ 8 (n = 134), and rSS > 8 (n = 68). There were significant differences in plaque characteristics among 3 groups (plaque rupture: 44.4% vs. 59.0% vs. 64.7%, the lowest to highest rSS, P = 0.040; OCT-defined high-risk plaques: 16.7% vs. 23.9% vs. 35.3%, the lowest to highest rSS, P = 0.036; calcification: 38.9% vs. 52.5% vs. 61.8%, the lowest to highest rSS, P = 0.024). During a mean follow-up of 2.2 years, MACE occurred in 47 patients (17.2%); rSS > 8 group had higher MACE risk compared to rSS = 0 (HR = 2.68, 95% CI: 1.11–6.5, P = 0.029). The study concluded that culprit plaque morphology was significantly correlated with rSS, and elevated rSS was associated with higher cardiovascular risk in STEMI patients.
Other studies on endovascular imaging Using wire-based index of microcirculatory resistance (IMR) as the reference standard, one study among 203 patients found that the overall diagnostic accuracy, sensitivity, specificity, positive predictive value and negative predictive value of the computational fluid dynamics-based AccuIMR for detecting coronary microvascular disease were 91.1%, 89.4%, 92.4%, 89.4%, and 92.2%, respectively.[27] The correlation coefficient equaled to 0.81 between AccuIMR and wire-based IMR with the receiver-operating curve had AUC of 0.924. The study suggested that the AccuIMR could be a valid, efficient, and cost-reducing tool to provide an easier routine assessment of coronary microcirculation.
Eighty-five patients with angina pectoris and no prior CAD history were included.[28] Myocardial work deriving from the left ventricular pressure-strain loop combined with treadmill exercise stress is a novel method for detecting significant CAD in patients with angina pectoris. Twenty-five patients had a positive exercise echocardiogram, while significant coronary artery stenosis was observed in 41 patients. The Global Wasted Work and Global Work Efficiency were significantly higher or lower, respectively, in patients with significant CAD compared with those of nonsignificant CAD at the peak exercise and during recovery periods (P < 0.05 for all). Multivariate logistic regression analysis demonstrated that peak Global Work Efficiency and recovery Global Wasted Work could detect significant CAD. Global myocardial work parameters combined with exercise stress perform as an accurate noninvasive screening before the invasive diagnostic technique.
AI improved the diagnostic accuracy and speed of CAD, which has favorable development prospects.
A retrospective study revealed that there was an excellent positive correlation between the automatic deep learning CTA and semiautomatic non-contrast CT coronary artery calcium score (Pearson correlation = 0.96; r2 = 0.92).[29] The risk categorization agreement was excellent (weighted κ = 0.94, 95% CI: 0.91–0.97). In conclusion, a deep learning automatic calcium scoring method accurately quantified coronary artery calcium from CT angiography images and categorized risk.
A study analyzed the data from 165 patients’ coronary CT angiography (680 vessels and 1505 segments).[30] The average post-processing and interpretation times of AI was 2.3 ± 0.6 min per case, reduced by 76%, 72%, 69% compared with low/ intermediate/ high experience readers (all P < 0.001), respectively. On a per-patient, per-vessel and per-segment basis, with invasive coronary angiography as reference standard, the AI overall diagnostic sensitivity for detecting obstructive CAD was 90.5%, 81.4%, 72.9%, the specificity was 82.3%, 93.9%, 95.0%, with the corresponding AUC of 0.90, 0.90, 0.87, respectively. Compared to human readers, the diagnostic performance of AI was higher than that of low experience readers (all P < 0.001). The diagnostic performance of AI + human reading was higher than human reading alone [Per-patient, the net reclassification improvement (NRI) = 0.085; per-vessel, NRI = 0.070; and per-segment, NRI = 0.068, all P < 0.001]. AI can substantially shorten the post-processing time, while AI + human reading model can significantly improve the diagnostic performance compared with human readers.
Standardization of strategy and management A sequential, explanatory mixed method study pointed out that for heart teams making vascular revascularization decisions in patients with complex CAD, attention should be paid to expert selection, expert training, team composition, team training, and meeting process.[31]
Improving Care for Cardiovascular Disease in China-Acute Coronary Syndrome (CCC-ACS) project evaluated the hospital performance in the management of STEMI by 9 strategies with class I recommendations in Chinese and United States guidelines. Participants included 57,560 patients with STEMI who were admitted to 143 tertiary hospitals across China from November 2014 to July 2019.[32] Results showed that the quality of care for these patients did not meet guideline-recommended strategies in China, with only 1 in 5 patients receiving all the care according to the 9 guideline-recommended strategies. Large disparities in the quality of care existed across hospitals.
A randomized study on mobile APP to improve medication adherence in patients with CAD showed that the mean decrease in medication non-adherence score was statistically significant at both 60 days (t179 = 2.04, P = 0.04) and 90 days (t155 = 3.48, P < 0.001).[33] Systolic blood pressure and diastolic blood pressure decreased in the experimental group but increased in the control group. The mean decrease in diastolic blood pressure was statistically significant at both 60 days (t160 = 2.07, P = 0.04) and 90 days (t164 = 2.21, P = 0.03). The proposed mobile health intervention can improve medication adherence and blood pressure.
Chest pain centre (CPC) accreditation A retrospective study using the data from the Hospital Quality Monitoring System (HQMS) included a total of 798,008 patients with ACS from 746 hospitals.[34] This study evaluated the impact of CPC accreditation on ACS emergency care and showed that patients with ACS admitted to accredited CPC had lower in-hospital mortality (OR = 0.70, 95% CI: 0.53–0.93), shorter length of stay (adjusted multiplicative effect = 0.89, 95% CI: 0.84–0.94) and more PCI procedures (OR = 3.53, 95% CI: 2.20–5.66) than patients admitted in hospitals without applying for CPC accreditation. The significant benefits of decreased in-hospital mortality, reduced length of stay and increased PCI usage were also observed for patients with AMI. The study proved that CPC accreditation was associated with better management and in-hospital clinical outcomes of patients with ACS. CPC establishment and accreditation should be promoted and implemented in countries with high prevalence of ACS.
Antiplatelet therapy A randomized controlled trial revealed that the addition of low-dose rivaroxaban to dual antiplatelet therapy (DAPT) reduced left ventricular thrombus formation within 30 days compared with only DAPT (0.7% vs. 8.6%; HR = 0.08, 95% CI: 0.01–0.62, P < 0.001 for superiority). Net clinical adverse events were lower within 30 days in the rivaroxaban group versus those in the only DAPT group and remained relatively low throughout the follow-up period. There were no significant differences in bleeding events between the two groups in 30 days and 180 days.[35]
A total of 127 patients were divided into two groups based on the duration of tirofiban use (less and more than 24 h) in a cohort study.[36] There was no significant difference between two groups in terms of baseline characteristics, plaque condition, and coronary physiological function. The two groups showed similar in-hospital MACE (1.85% vs. 5.48%, P = 0.394) and 1-year MACE-free survival (P = 0.9085). The 1-year MACE remained consistent between the two groups in all subgroups of different risk factors of no-/slow-reflow. There was no significant difference in heart function and in-hospital hemorrhage events (3.7% vs. 1.37%, P = 0.179). The study concluded that in the real world, prolonging the duration of GP IIb/IIIa inhibitor could not significantly improve the clinical outcomes in patients with STEMI with no-/slow-reflow.
A multi-center, randomized, double-blind clinical trial showed that intravenous administration of the platelet receptor GP Ib antagonist anfibatide was feasible and safe to inhibit platelet aggregation without increasing the risk of bleeding and thrombocytopenia in patients with NSTEMI undergoing PCI.[37]
A multi-center, randomized, double-blind, triple-dummy, dose-exploring phase II trial evaluated the effect and safety of novel antiplatelet prodrug vicagrel in patients with CAD.[38] The study proved that vicagrel had comparable antiplatelet efficacy (inhibition of platelet aggregation rate) and safety (plasma concentration of metabolite and bleeding) to clopidogrel in patients with CAD undergoing PCI.
PATH-PCI trial was a randomized, controlled, open-label trial. The study suggested that compared with standard DAPT treatment with no platelet function testing, the personalized antiplatelet therapy guided by a novel platelet function testing could reduce ischemic events both in diabetes group (6.8% vs. 11.3%, HR = 0.586, 95% CI: 0.344–0.999, P = 0.049) and in nondiabetic group (1.8% vs. 4.2%, HR = 0.428, 95% CI: 0.233–0.758, P = 0.006), but not increase the risk of bleeding in stable CAD patients with or without diabetes who have undergone PCI.[39]
Anticoagulant therapy A total of 918 female patients from 27 Chinese medical centers with bivalirudin as anticoagulant for PCI were enrolled in a multi-center trial.[40] Safety data were collected from admission to 72 h post bivalirudin administration and at the 30th day after follow-up. The study demonstrated that bivalirudin was well tolerated with low adverse events, thrombocytopenia and bleeding incidences.
CCC-ACS project assessed the association between postprocedural anticoagulation use and several clinical outcomes among patients with STEMI undergoing PCI.[41] Of 34,826 evaluable patients, 26,272 patients (75.4%) were treated with postprocedural anticoagulation. The study found out that postprocedural anticoagulation was associated with significantly reduced risk of in-hospital mortality (0.9% vs. 1.8%, HR = 0.62; 95% CI: 0.43–0.89, P < 0.001) and a nonsignificant difference in risk of in-hospital major bleeding (2.5% vs. 2.2%, HR = 1.05; 95% CI: 0.83–1.32, P = 0.14).
Other treatment CCC-ACS project revealed that compared with non-users, early (within the first 24 h) oral beta-blocker therapy was associated with reduced risk for major bleeding (OR = 0.48; 95% CI: 0.38–0.61) and in-hospital mortality (OR = 0.47; 95% CI: 0.34–0.64) in multivariable-adjusted logistic regression models. Analyses based on inverse probability of treatment-weighted regression adjustment and propensity-score matching yielded consistent findings.[42]
CCC-ACS project also evaluated the association between proton pump inhibitor (PPI) use and in-hospital gastrointestinal bleeding in 25,567 patients with ACS taking DAPT from 172 hospitals.[43] The results showed that in patients with ACS, 63.9% were prescribed PPI within 24 h of admission. Patients using PPI had a higher risk of gastrointestinal bleeding compared with those not using PPI (HR = 1.58; 95% CI: 1.15–2.18, P = 0.005).
New developments in instruments and techniques In a multi-center randomized controlled trial in China, 212 patients with small-vessel native coronary disease (reference vessel diameter: 2.0–2.75 mm, lesion length ≤ 25 mm) were randomized to receive a domestic biolimus-coated balloon (BCB) or an uncoated balloon.[44] In the per-protocol population, angiographic late lumen loss at 9 months was 0.16 ± 0.29 mm in the BCB group versus 0.30 ± 0.35 mm in the plain balloon group (P = 0.001). Late luminal enlargement (positive remodeling) occurred in 29.7% of patients in the BCB group versus 9.8% of patients in the plain balloon group. In the full analysis set population, after 12 months, target lesion failure (TLF) rates were 6.7% in the BCB group versus 13.9% in the plain balloon group (HR = 0.47; 95% CI: 0.19–1.16), and rates of the patient-oriented clinical outcome were 14.3% with the BCB versus 21.8% with the plain balloon (HR = 0.64; 95% CI: 0.33–1.24).
TARGET STEMI OCT China trial compared the vascular healing of a biodegradable polymer sirolimus-eluting stent (Firehawk) versus a durable polymer everolimus-eluting stent (Xience) after PCI within 12 h of STEMI onset.[45] The results showed that the Firehawk stent was non-inferior to the Xience stent in terms of the neointimal thickness [73.03 ± 33.30 μm vs. 78.96 ± 33.29 μm; absolute difference: -5.94 (one-sided 95% CI: -23.09); P < 0.001 for non-inferiority] at 6 months. No significant difference was observed between the Firehawk and Xience regarding the strut coverage, neointimal formation, and strut malapposition. At 12 months, one patient in the Firehawk group experienced a target lesion revascularization. No other TLF events occurred in both groups. The trial suggested that in patients with STEMI, Firehawk was non-inferior to Xience in vascular healing at 6 months.
A first-in-human study of the newer generation bioresorbable scaffold with 2-year follow-up revealed that among 30 patients, one patient had definite scaffold thrombosis within 6 months of follow-up.[46] Imaging follow-up was available in 24 patients, and in-scaffold late loss was 0.44 ± 0.47 mm. Intracoronary imaging confirmed the late loss was mainly due to neointimal hyperplasia, but not scaffold recoil. The results confirmed the preliminary safety and efficacy of bioresorbable scaffold for treatment of simple coronary lesions.
One multi-center, prospective, observational study enrolled 119 patients with de novo coronary lesions in vessels ≥ 2.75 mm. The long-term clinical outcomes of drug-coated balloon-only strategy for the treatment of de novo bifurcation and non-bifurcation lesions in large coronary arteries were assessed. Patients were followed up for a median of 2 years.[47] The frequency of target lesion revascularization and target vessel revascularization was higher in the non-bifurcation group (P = 0.04 and 0.02, respectively), but there was no difference in TLF between the two groups (P = 0.17). The cumulative incidence of TLF was also not different in the two groups. Drug-coated balloon-only strategy for de novo lesions in large coronary arteries appears to be safe and effective for both bifurcation and non-bifurcation lesions.
New progress in clinical studies Predictors of success in reattempted chronic total occlusion (CTO) PCI procedures remain obscure, mainly owing to the lack of consecutive angiograms and procedural records of initial attempts in the same cohort. A total of 208 consecutive patients who underwent a failed CTO PCI attempt and received reattempted procedure were retrospectively analyzed. Predictors of the success of reattempted procedures were evaluated.[48] The study identified that subintimal plaque modification with guidewire crossing, referral to high-volume operators, and a bidirectional approach were positive independent predictors of technical success in the subsequent reattempt. The time interval for reattempt was negatively correlated with the technical success of the reattempted procedures.
Based on the data of CAMI registry, one study aimed to determine whether late PCI of an infarct-related artery > 12 h after STEMI onset was beneficial.[49] Compared with drug therapy, PCI was associated with lower incidences of 2-year major adverse cardiac and cerebrovascular event (MACCE), all-cause death, myocardial infarction, stroke, and revascularization. Subgroup analysis consistently indicated that PCI was superior to drug therapy. Moreover, the left ventricular ejection fraction in the PCI group was increased after 2-year follow-up, whereas there was no significant increase in the drug therapy group. Late PCI is common in Chinese clinical practice, and it is associated with significant improvements in cardiac function and survival rate compared with drug therapy alone.
A study compared the long-term clinical outcomes and health status of PCI for patients within-stent coronary CTO (IS-CTO) versus patients with de novo coronary CTO (de novo CTO) in the drug-eluting stent (DES) era.[50] A total of 483 consecutive patients with 1 CTO lesion were screened. The success rates of CTO lesion revascularization were similar in both groups. After a median follow-up of 36 months, MACE was observed in 32.8% of patients with IS-CTO versus 13.5% of the patients with de novo CTO (P < 0.001), mainly driven by target-vessel revascularization (21.9% vs. 6.7%, P < 0.01). Moreover, patients with IS-CTO had significantly worse Seattle Angina Questionnaire angina stability scores than the patients with de novo CTO. In conclusion, patients with IS-CTO after PCI had a worse clinical outcome.
Numbers of PCI in China In 2021, 1,164,117 patients were registered to be treated with PCI in China (excluding those in military hospitals), which increased by 14.77% in comparison with the estimate in 2020 (Figure 12). The average number of stent or drug-eluting balloon was 1.48 per patient which has remained stable at less than 1.5 since 2014. The proportion of patients who were treated with drug-eluting balloon for PCI increased progressively from 2019 to 2021, with the estimates of 6.4%, 10.9%, and 15% for the 3 years, respectively. The mortality of PCI has maintained at a low level since 2019. It was 0.38% in 2021, same as that in 2020.
In terms of clinical diagnosis of PCI, unstable angina pectoris had the highest proportion (42.79%), followed by STEMI (22.52%), stable angina pectoris (14.19%) and NSTEMI (13.20%). Suspected angina pectoris and silent myocardial ischemia accounted for 5.31% and 1.99%, respectively (Figure 13).
New progress in surgical techniques and instruments Vein graft occlusion is deemed a major challenge in coronary artery bypass grafting (CABG). Previous studies implied that the no-touch technique for vein graft harvesting could reduce occlusion rate compared with the conventional approach; however, evidence on the clinical benefit of the no-touch technique is scare. In a randomized controlled study, 2655 patients undergoing CABG were randomly assigned in a 1:1 ratio to receive no-touch technique or conventional approach for vein harvesting.[51] The no-touch group had significantly lower rates of vein graft occlusion than the conventional group both at 3 months (2.8% vs. 4.8%) and 12 months (3.7% vs. 6.5%). Recurrence of angina was also less common in the no-touch group at 12 months (2.3% vs. 4.1%). Rates of MACCE were of no significant difference between the 2 groups. The no-touch technique was associated with higher rates of leg wound surgical interventions at 3 months (10.3% vs. 4.3%).
The Surgical Treatment for Ischemic Heart Failure (STICH) trial revealed that in patients with ischemic cardiomyopathy, off-pump CABG was associated with a higher prevalence of multiple arterial grafting (17.1% vs. 8.6%, P = 0.01) and incomplete revascularization (34.2% vs. 17.1%, P < 0.001) in comparison with on-pump CABG. The overall 30-day mortality (3.3% vs. 5.3%, P = 0.34) was comparable between the 2 groups. After a median follow-up of 8.7 years, a similar risk of death from any cause was observed between the 2 groups (HR = 0.82, 95% CI: 0.61–1.09).[52]
A first-in-man investigation of an implantable Heartech® left ventricular partitioning device therapy for chronic HF after myocardial infarction recruited 16 patients.[53] During 1-year follow-up, 1 patient suffered a fatal myocardial infarction, other patients did not report any MACCE. After the operation, the average left ventricular end-systolic volume index decreased dramatically (66.00 mL/m2 vs. 48.00 mL/m2, P = 0.001), along with left ventricular end-diastolic volume index (105.00 mL/m2 vs. 76.50 mL/m2, P = 0.002). The left ventricular ejection fraction improved markedly (35.00% vs. 42.50%, P = 0.003). The study suggested that the Heartech® left ventricular partitioning device was safe and effective in reducing left ventricular volume, enhancing left ventricular function after implantation.
One study sought to compare postoperative bleeding and renal function in patients with multivessel CAD undergoing simultaneous hybrid coronary revascularization (HCR) and minimally invasive direct off-pump CABG (MIDCABG).[54] The study collected the data of 594 consecutive patients who underwent simultaneous HCR and 351 patients who underwent MIDCABG. A total of 317 pairs of patients who were matched in a 1:1 ratio with propensity score matching were enrolled in this study. Compared with patients who underwent MIDCABG, patients who underwent simultaneous HCR had significantly greater chest tube drainage on the day of the operation (492.7 ± 282.4 mL vs. 441.0 ± 261.9 mL, P = 0.023), but no significant difference was detected in the total amount during the postoperative period. The differences in repeated revascularization, blood transfusion, and in-hospital MACCE between the 2 groups did not reach statistical significance.
Comparison of intervention therapy and CABG One single-center study investigated 5-year clinical outcomes (composite of death, myocardial infarction, or stroke) following CABG and PCI in patients with CTO and multivessel disease.[55] The unadjusted 5-year composite outcomes were similar between the CABG group and the PCI group. After adjustment for baseline variables, PCI was associated with significantly higher risk of composite outcomes (HR = 1.21, 95% CI: 1.02–1.44). The inferiority of PCI in 5-year composite outcome was significant in patients with CABG recommendation according to SYNTAX score II (HR = 1.55, 95% CI: 1.14–2.09) but not evident in patients with PCI or PCI/CABG equipoise recommendation according to SYNTAX score II (HR = 0.94, 95% CI: 0.75–1.17). A similar risk of 5-year composite outcomes was observed between CABG and PCI with residual SYNTAX score ≤ 8.
The best revascularization strategy for patients with ischemic HF remains unclear. One study was designed to compare clinical outcomes of CABG with implantation of DES in patients with mild to moderate ischemic HF (ejection fraction: 35%–50%). A total of 2050 patients were included with median follow-up of 45 months.[56] After propensity score matching for the entire population, the long-term cumulative rate of all-cause death was significantly different between the two groups (DES vs. CABG: 5.8% vs. 2.7%, P = 0.006). The rate of repeat coronary revascularization was significantly higher in the DES group than in the CABG group (12.1% vs. 6.0%, P = 0.000). No differences were found in the rates of cardiac death, recurrent myocardial infarction, and stroke between the two groups. The study suggests that CABG is superior to DES implantation in the patients with mildly to moderately reduced ejection fraction and significant CAD.
One study was designed to compare the real-world outcomes of medical therapy, CABG and PCI treatment strategies in patients with diabetes and triple-vessel disease (TVD).[57] The intermediate-term follow-up results showed that PCI and CABG were associated with a lower risk of death and MACCE in comparison with medical therapy. The results suggested the importance of appropriate revascularization for diabetic patients with TVD. However, CABG was not associated with a lower risk of death, but with a lower risk of MACCE, compared with PCI. In the future, comprehensive treatment in addition to PCI or CABG should be strengthened in diabetic patients with TVD.
One prospective study compared the effect of PCI and CABG surgery in patients with triple-vessel CAD, HF, and different degrees of mitral regurgitation (MR).[58] In this real-world study, for patients with HF and TVD, CABG was related to lower adverse outcome rates compared with PCI, especially in patients with moderate MR. Assessment of MR can aid in selecting optimal revascularization therapies and in risk stratification.
Perioperative management and medical therapy of CABG One prospective study evaluated the effect of clopidogrel within 5 days before CABG on outcomes in patients with ACS.[59] The results revealed that patients with ACS undergoing CABG, clopidogrel therapy within 5 days before surgery was associated with increased odds of MACCE and bleeding complications than discontinuing clopidogrel for > 5 days.
A total of 42,010 patients undergoing CABG were enrolled in a retrospective cohort study.[60] Tranexamic acid (TXA) led to a 1.40-fold risk of perioperative myocardial infarction (P < 0.001). Patients in the TXA group had fewer re-operations for bleeding or cardiac tamponade (OR = 0.82, P = 0.044), less blood loss after surgery (P < 0.001), and a lower risk for blood transfusion exposure (OR = 0.45, P < 0.001) than those in the no-TXA group. The high-dose TXA reduced blood loss after cardiac surgery compared to the low-dose TXA with no associations with blood exposure or adverse events. In conclusion, this study showed that the use of TXA during surgery increased the thromboembolic risk for patients undergoing CABG, despite better bleeding control.
Outcomes and quality control of surgical treatment The change in CABG performance after years of quality improvement measures was investigated in a national registry study in China.[61] The study included 66,971 patients who underwent isolated CABG in a cohort of 74 tertiary hospitals in China between January 2013 and December 2018. Data were collected from the Chinese Cardiac Surgery Registry. The in-hospital mortality declined from 0.9% in 2013 to 0.6% in 2018, with a risk-adjusted odds ratio of 0.66 (95% CI: 0.46–0.93, P < 0.001). The inter-hospital heterogeneity in patient outcome after CABG has been significantly reduced. The prevalence of adherence to guideline recommendations on evidence-based surgical process and secondary prevention progressively increased.
One retrospective cohort study compared the outcomes between CABG performed first versus those performed after prior procedures.[62] Patients were categorized as undergoing on-pump and off-pump CABG based on the difference in technical complexity. The study found out that in the on-pump cohort, there was no significant association between procedure order and the outcomes. In the off-pump cohort, non-first procedures were associated with an increased number of adverse events composite (adjusted RR = 1.29, 95% CI: 1.13–1.47, P < 0.001), myocardial infarction and stroke compared with first procedures. These increases were only found to be statistically significant when the procedure was performed by surgeons with < 20 years’ practice or surgeons with a preindex volume < 700 cases.
Risk prediction model 10-year CVD risk prediction model developed by Chinese involved 489,596 individuals, including 45,947 IHD, 43,647 ischemic stroke, and 11,168 hemorrhage stroke cases during 11 years of follow-up.[63] This CVD risk prediction model yielded good discrimination of IHD and stroke subtypes in addition to total CVD without including blood lipid. Flexible recalibration of the model for different regions could enable more widespread utility using resident health records covering the overall Chinese population.
A study analyzed the polygenic risk score (PRS) of CAD related features in the Chinese populations, and found that the highest 20% of PRS patients had a risk of developing CAD about 3 times higher than the lowest 20% (HR = 2.91, 95% CI: 2.43–3.49).[64] The study implied that PRS could stratify individuals into different trajectories of CAD risk, and further refine risk stratification for CAD within each clinical risk strata, demonstrating a great potential to identify high-risk individuals for targeted intervention in clinical utility.
Outcome prediction A total of 10,724 patients who underwent PCI were followed up for 5 years in a prospective study.[65] The PRECISE-DAPT score was calculated based on age, hemoglobin, white cell, creatinine clearance rate and bleeding history. The bleeding endpoint was Bleeding Academic Research Consortium (BARC) 2, 3, or 5 bleeding. The ischemic endpoints were all-cause death and MACCE. The study results revealed that the PRECISE-DAPT score had a statistically significant predictive value for 5-year bleeding events, and also some prognostic value for death and MACCE in the Chinese PCI population.
Stress hyperglycemia is a strong predictor of adverse outcomes in patients with AMI. One study involving 7476 acute STEMI patients revealed that the stress hyperglycemia ratio was independently related to the risks of MACE and mortality in patients with STEMI. Furthermore, the stress hyperglycemia ratio may aid in improving the predictive efficiency of the thrombolysis in myocardial infarction risk score in patients with STEMI, especially those with diabetes.[66]
A large-scale cohort study showed that routine detection of creatine kinase-myocardial band following CTO PCI was prognostically significant and may be helpful for risk stratification and treatment decision-making of high-risk patients.[67]
Triglyceride glucose (TyG) index is a simple and reliable surrogate marker of insulin resistance, which has been proved to be an independent predictor of long-term MACE, regardless of diabetes mellitus. A retrospective cohort study enrolled 986 ACS patients undergoing PCI. The analysis suggested that TyG index was an independent predictor of long-term MACE after PCI in all types of ACS patients, and improved the ability of the GRACE (Global Registry of Acute Coronary Events) score to stratify risk and predict prognosis of ACS patients undergoing PCI.[68] In addition, one retrospective study was designed to identify the potential association between insulin resistance quantified by TyG index and clinical prognosis in patients with non-ST-segment elevation ACS (NSTE-ACS). The study results revealed that the increased TyG index was a significant predictor of adverse prognosis in patients with type 2 diabetes mellitus and NSTE-ACS undergoing PCI.[69] The addition of TyG index to a baseline risk model had an incremental effect on the predictive value of adverse prognosis. Another study enrolled 10,535 CAD patients and evaluated the correlation between TyG index and carotid artery plaque. The study revealed a significant association between TyG index and carotid artery plaque in CAD patients. The association was higher in females than in males, and higher in middle-aged patients than in elderly patients. In the condition of diabetes, the TyG index has the highest correlation with carotid plaque.[70]
Risk factors Lifestyle has an important impact on cardiovascular health. Night shift is getting more and more attention in recent years. One study showed that disruption of circadian rhythms by shift work exacerbated reperfusion injury in myocardial infarction. During a median follow-up of 5 years, shift work was associated with increased risk of MACE.[71] Apart from shift work, physical activity (PA) is also associated with the risk of CVD. During a mean follow-up of 8.9 years, participants in the high PA trajectory group had a higher risk of coronary artery calcium progression than those in the low PA trajectory group. However, high PA trajectory was notassociated with an increased risk of incident CVD events.[72] A prospective observational study in China demonstrated that a paradoxical finding in the number of standard modifiable risk factors (SMuRFs) and mortality could be explained by confounding factors related to their poor risk profiles (older age, and poorer clinical management) among patients without SMuRFs. After multivariate adjustment, a higher risk-factor burden was associated with poor prognosis among patients with STEMI.[73] Type D personality includes a series of negative emotional factors and social resistance. Previous studies have shown that type D personality can predict MACE in CAD patients. A prospective observational study published in 2022 showed that type D personality was also an independent predictor of adverse outcomes in patients with AMI.[74].
The CCC-ACS project showed that the incidence of in-hospital major bleeding in ACS patients after treatment decreased from 6.3% in 2015 to 4.7% in 2019 (unadjusted OR = 0.74, 95% CI: 0.68–0.80, P < 0.001). The relative changes were consistent across almost all subgroups including patients with NSTE-ACS and STEMI. The decrease in bleeding was accompanied by a decrease in use of GP IIb/IIIa inhibitors and parenteral anticoagulation therapy during hospitalization. The annual reduced risk of bleeding was attenuated after stepwise adjusting for baseline characteristics and antithrombotic treatments, but did not change after adjusting for invasive treatment.[75]
A cohort study of Chinese adults examined the relationship between IHD and education attainment. The study included 489,594 participants in the baseline China Kadoorie Biobank survey from 2004 to 2008, with a median follow-up of 11.1 years.[76] The study found out that lower educational attainment was associated with increased risk of incident AMI as well as death and fatality of total IHD (Ptrend < 0.001). Smoking and dietary habits were the two most potent mediating factors in the associations of education with mortality and AMI incidence, PA was the major mediating factor for non-AMI incidence in the whole population. Interventions targeting unhealthy lifestyles are ideal ways to narrow the educational gap in incident IHD.
CAD is an important cardiovascular disease that threatens the health of Chinese residents, and its overall prevalence and mortality are on the rise. Technological innovation and achievement transformation are becoming the main trends in the field of CAD diagnosis and treatment. In terms of imageology, multiple domestic teams have taken the lead in proposing functional indicators such as QFR and CT-FFR, conducting research based on intracavitary imaging, providing new methods for quickly and safely evaluating the anatomical and hemodynamic information of coronary stenosis. AI can improve the diagnostic accuracy and speed of CAD, and has a promising future. Drug therapy is an indispensable and important treatment option for CAD, and multiple clinical trials have made progress on antiplatelet and anticoagulant therapy. In the field of surgical treatment, multiple domestically produced interventional treatment instruments and surgical procedures have taken the world’s leading position. Although remarkable progress has been made in the treatment of CAD and AMI, the standardization of treatment still needs to be improved in China. The best treatment strategies for patients with CAD are also constantly being explored, and continuous research on prognostic predictors and risk factors will lay a solid foundation for prevention and treatment of CAD in the future.
| [1] |
National Health Commission of PR China. China Health and Health Statistics Yearbook 2021; China Union Medical University Press, Beijing, China: 2021.
|
| [2] |
Wang W, Liu YN, Liu JM, et al. Mortality and years of life lost of cardiovascular diseases in China, 2005–2020: empirical evidence from national mortality surveillance system. Int J Cardiol 2021; 340: 105−112. doi: 10.1016/j.ijcard.2021.08.034
|
| [3] |
Zhao D, Liu J, Wang M, et al. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol 2019; 16: 203−212. doi: 10.1038/s41569-018-0119-4
|
| [4] |
Institute for Health Metrics and Evaluation (IHME). GBD results tool. 2019. IHME Website. http://ghdx.healthdata.org/gbd-results-tool.
|
| [5] |
Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, et al. Myocardial infarction and coronary deaths in the World Health Organization MONICA project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation 1994; 90: 583−612. doi: 10.1161/01.cir.90.1.583
|
| [6] |
Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health 2013; 1: e259−e281. doi: 10.1016/S2214-109X(13)70089-5
|
| [7] |
Zhang GS, Yu CH, Zhou MG, et al. Burden of ischaemic heart disease and attributable risk factors in China from 1990 to 2015: findings from the global burden of disease 2015 study. BMC Cardiovasc Disord 2018; 18: 18. doi: 10.1186/s12872-018-0761-0
|
| [8] |
Wang WZ, Jiang B, Sun HX, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480, 687 adults. Circulation 2017; 135: 759−771. doi: 10.1161/CIRCULATIONAHA.116.025250
|
| [9] |
Moran A, Gu DF, Zhao D, et al. Future cardiovascular disease in China: Markov model and risk factor scenario projections from the Coronary Heart Disease Policy Model-China. Circ Cardiovasc Qual Outcomes 2010; 3: 243−252. doi: 10.1161/CIRCOUTCOMES.109.910711
|
| [10] |
Moran A, Zhao D, Gu DF, et al. The future impact of population growth and aging on coronary heart disease in China: projections from the Coronary Heart Disease Policy Model-China. BMC Public Health 2008; 8: 394. doi: 10.1186/1471-2458-8-394
|
| [11] |
National Health Commission of PR China. Analysis Report of the Fifth National Health Service Survey in 2013. China Union Medical University Press, Beijing, China: 2021.
|
| [12] |
National Health Commission of PR China. China Health Statistics Yearbook 2021. China Union Medical University Press, Beijing, China: 2021.
|
| [13] |
Li J, Li X, Wang Q, et al. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet 2015; 385: 441−451. doi: 10.1016/S0140-6736(14)60921-1
|
| [14] |
Zhang Q, Zhao D, Xie WX, et al. Recent trends in hospitalization for acute myocardial infarction in Beijing: increasing overall burden and a transition from ST-segment elevation to non-ST-segment elevation myocardial infarction in a population-based study. Medicine (Baltimore) 2016; 95: e2677. doi: 10.1097/MD.0000000000002677
|
| [15] |
Zhao QH, Yang YJ, Chen ZJ, et al. Changes in characteristics, risk factors, and in-hospital mortality among patients with acute myocardial infarction in the capital of China over 40 years. Int J Cardiol 2018; 265: 30−34. doi: 10.1016/j.ijcard.2018.04.134
|
| [16] |
Xu HY, Yang YJ, Wang CS, et al. Association of hospital-level differences in care with outcomes among patients with acute ST-segment elevation myocardial infarction in China. JAMA Netw Open 2020; 3: e2021677. doi: 10.1001/jamanetworkopen.2020.21677
|
| [17] |
Zhao YY, Yang JG, Xu HB, et al. Correlation analysis of medical quality and in-hospital mortality of acute ST-segment elevation myocardial infarction in China. Chin Circ J 2019; 34: 437−443.
|
| [18] |
Song CX, Fu R, Yang JG, et al. The association between body mass index and in-hospital outcome among patients with acute myocardial infarction: insights from China Acute Myocardial Infarction (CAMI) registry. Nutr Metab Cardiovasc Dis 2019; 29: 808−814. doi: 10.1016/j.numecd.2019.04.001
|
| [19] |
He L, Lu F, Du X, et al. Impact of COVID-19 pandemic on hospital admissions of acute coronary syndrome: a Beijing inpatient database study. Lancet Reg Health West Pac 2022; 19: 100335. doi: 10.1016/j.lanwpc.2021.100335
|
| [20] |
Luo S, Qiu XM, Zeng XJ, et al. Coronary artery calcification and risk of mortality and adverse outcomes in patients with COVID-19: a Chinese multicenter retrospective cohort study. Chin J Acad Radiol 2022; 5: 20−28. doi: 10.1007/s42058-021-00072-4
|
| [21] |
Xu B, Tu SX, Song L, et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre, randomised, sham-controlled trial. Lancet 2021; 398: 2149−2159. doi: 10.1016/S0140-6736(21)02248-0
|
| [22] |
Zhang R, Dou KF, Guan CD, et al. Outcomes of quantitative flow ratio-based percutaneous coronary intervention in an all-comers study. EuroIntervention 2022; 17: 1240−1251. doi: 10.4244/EIJ-D-21-00176
|
| [23] |
Liu X, Mo XK, Zhang HY, et al. A 2-year investigation of the impact of the computed tomography-derived fractional flow reserve calculated using a deep learning algorithm on routine decision-making for coronary artery disease management. Eur Radiol 2021; 31: 7039−7046. doi: 10.1007/s00330-021-07771-7
|
| [24] |
Yu YR, Yu LH, Dai X, et al. CT fractional flow reserve for the diagnosis of myocardial bridging-related ischemia: a study using dynamic CT myocardial perfusion imaging as a reference standard. Korean J Radiol 2021; 22: 1964−1973. doi: 10.3348/kjr.2021.0043
|
| [25] |
Wang YB, Chen HW, Sun T, et al. Risk predicting for acute coronary syndrome based on machine learning model with kinetic plaque features from serial coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 2022; 23: 800−810. doi: 10.1093/ehjci/jeab101
|
| [26] |
Wang Y, Zhao XX, Zhou P, et al. Residual SYNTAX score in relation to coronary culprit plaque characteristics and cardiovascular risk in ST-segment elevation myocardial infarction: an intravascular optical coherence tomography study. J Cardiovasc Transl Res 2022; 15: 75−83. doi: 10.1007/s12265-021-10152-6
|
| [27] |
Jiang J, Li CG, Hu YM, et al. A novel CFD-based computed index of microcirculatory resistance (IMR) derived from coronary angiography to assess coronary microcirculation. Comput Methods Programs Biomed 2022; 221: 106897. doi: 10.1016/j.cmpb.2022.106897
|
| [28] |
Lin JR, Wu WC, Gao LJ, et al. Global myocardial work combined with treadmill exercise stress to detect significant coronary artery disease. J Am Soc Echocardiogr 2022; 35: 247−257. doi: 10.1016/j.echo.2021.10.009
|
| [29] |
Mu D, Bai JJ, Chen WP, et al. Calcium scoring at coronary CT angiography using deep learning. Radiology 2022; 302: 309−316. doi: 10.1148/radiol.2021211483
|
| [30] |
Liu CY, Tang CX, Zhang XL, et al. Deep learning powered coronary CT angiography for detecting obstructive coronary artery disease: the effect of reader experience, calcification and image quality. Eur J Radiol 2021; 142: 109835. doi: 10.1016/j.ejrad.2021.109835
|
| [31] |
Ma HP, Lin S, Li X, et al. Optimal heart team protocol to improve revascularization decisions in patients with complex coronary artery disease: a sequential mixed method study. Eur Heart J Qual Care Clin Outcomes 2022; 8: 739−749. doi: 10.1093/ehjqcco/qcab074
|
| [32] |
Hao YC, Zhao D, Liu J, et al. Performance of management strategies with class I recommendations among patients hospitalized with ST-segment elevation myocardial infarction in China. JAMA Cardiol 2022; 7: 484−491. doi: 10.1001/jamacardio.2022.0117
|
| [33] |
Ni Z, Wu B, Yang Q, et al. An mHealth intervention to improve medication adherence and health outcomes among patients with coronary heart disease: randomized controlled trial. J Med Internet Res 2022; 24: e27202. doi: 10.2196/27202
|
| [34] |
Sun PF, Li JP, Fang WY, et al. Effectiveness of chest pain centre accreditation on the management of acute coronary syndrome: a retrospective study using a national database. BMJ Qual Saf 2021; 30: 867−875. doi: 10.1136/bmjqs-2020-011491
|
| [35] |
Zhang ZF, Si DY, Zhang Q, et al. Prophylactic rivaroxaban therapy for left ventricular thrombus after anterior ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2022; 15: 861−872. doi: 10.1016/j.jcin.2022.01.285
|
| [36] |
Hu X, Wang W, Ye J, et al. Effect of GP IIb/IIIa inhibitor duration on the clinical prognosis of primary percutaneous coronary intervention in ST-segment elevation myocardial infarction with no-/slow-reflow phenomenon. Biomed Pharmacother 2021; 143: 112196. doi: 10.1016/j.biopha.2021.112196
|
| [37] |
Zheng B, Li JP, Jiang J, et al. Safety and efficacy of a platelet glycoprotein Ib inhibitor for patients with non-ST-segment elevation myocardial infarction: a phase Ib/IIa study. Pharmacotherapy 2021; 41: 828−836. doi: 10.1002/phar.2620
|
| [38] |
Zhao X, Ma SC, Kang Y, et al. Antiplatelet effect, safety, and pharmacokinetics of vicagrel in patients with coronary artery disease undergoing percutaneous coronary intervention. Eur Heart J Cardiovasc Pharmacother 2022; 8: 806−814. doi: 10.1093/ehjcvp/pvac026
|
| [39] |
Zheng YY, Wu TT, Yang Y, et al. Diabetes and outcomes following personalized antiplatelet therapy in coronary artery disease patients who have undergone PCI. J Clin Endocrinol Metab 2022; 107: e214−e223. doi: 10.1210/clinem/dgab612
|
| [40] |
Wu F, Liu X, Ran H, et al. Safety profile of bivalirudin in Chinese female patients undergoing percutaneous coronary intervention: a multi-center study. BMC Cardiovasc Disord 2022; 22: 58. doi: 10.1186/s12872-022-02474-3
|
| [41] |
Yan Y, Gong W, Ma CS, et al. Postprocedure anticoagulation in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. JACC Cardiovasc Interv 2022; 15: 251−263. doi: 10.1016/j.jcin.2021.11.035
|
| [42] |
Xu S, Li Z, Yang T, et al. Association between early oral β-blocker therapy and risk for in-hospital major bleeding after percutaneous coronary intervention for acute coronary syndrome: findings from CCC-ACS project. Eur Heart J Qual Care Clin Outcomes 2022; 17: qcac036. doi: 10.1093/ehjqcco/qcac036
|
| [43] |
Zhou MG, Zhang J, Liu J, et al. Proton pump inhibitors and in-hospital gastrointestinal bleeding in patients with acute coronary syndrome receiving dual antiplatelet therapy. Mayo Clin Proc 2022; 97: 682−692. doi: 10.1016/j.mayocp.2021.11.037
|
| [44] |
Xu K, Fu GS, Tong Q, et al. Biolimus-coated balloon in small-vessel coronary artery disease: the BIO-RISE CHINA study. JACC Cardiovasc Interv 2022; 15: 1219−1226. doi: 10.1016/j.jcin.2022.03.024
|
| [45] |
He Y, Wang RT, Liu JZ, et al. A randomized comparison of the healing response between the Firehawk stent and the Xience stent inpatients with ST-segment elevation myocardial infarction at 6 months of follow-up (TARGET STEMI OCT China Trial): an optical coherence tomography study. Front Cardiovasc Med 2022; 9: 895167. doi: 10.3389/fcvm.2022.895167
|
| [46] |
Ferdous MM, Jie Z, Gao L, et al. A first-in-human study of the Bioheart sirolimus-eluting bioresorbable vascular scaffold in patients with coronary artery disease: two-year clinical and imaging outcomes. Adv Ther 2022; 39: 3749−3765. doi: 10.1007/s12325-022-02154-w
|
| [47] |
Hu FW, Chang S, Li Q, et al. Long-term clinical outcomes after percutaneous coronary intervention with drug-coated balloon-only strategy in lesions of large coronary arteries. Front Cardiovasc Med 2022; 9: 882303. doi: 10.3389/fcvm.2022.882303
|
| [48] |
Zhong X, Gao W, Hu T, et al. Impact of subintimal pl aque modification on reattempted chronic total occlus ions percutaneous coronary intervention. JACC Cardio vasc Interv 2022; 15: 1427−1437. doi: 10.1016/j.jcin.2022.06.015
|
| [49] |
Hu MJ, Peng Y, Gao XJ, et al. Coronary intervention in ST-segment elevation myocardial infarction patients with symptom onset > 12 hours: data from China Acute Myocardial Infarction registry. Angiology 2023; 74: 171−180. doi: 10.1177/00033197221098885
|
| [50] |
Gao K, Li BL, Zhang M, et al. Long-term outcomes of percutaneous coronary intervention for patients within-stent chronic total occlusion versus de novo chronic total occlusion. Angiology 2021; 72: 740−748. doi: 10.1177/0003319721998575
|
| [51] |
Tian MC, Wang XQ, Sun HS, et al. No-touch versus conventional vein harvesting techniques at 12 months after coronary artery bypass grafting surgery: multicenter ra ndomized, controlled trial. Circulation 2021; 144: 1120−1129. doi: 10.1161/CIRCULATIONAHA.121.055525
|
| [52] |
Zhou ZM, Liang MY, Zhuang XD, et al. Long-term outcomes after on-pump vs off-pump coronary artery bypass grafting for ischemic cardiomyopathy. Ann Thorac Surg 2023; 115: 1421−1428. doi: 10.1016/j.athoracsur.2021.12.063
|
| [53] |
Zhu ZB, Zhu JZ, Yu JW, et al. Percutaneous ventricular restoration prevents left ventricular remodeling post myocardial infarction: one-year evaluation of the Heartech first-in-man study. J Card Fail 2022; 28: 604−613. doi: 10.1016/j.cardfail.2021.10.017
|
| [54] |
Ding T, Hu SS, Qu JY, et al. Evaluation of the effect of simultaneous hybrid coronary revascularization on postoperative bleeding and renal function: a comparison study with minimally invasive direct off-pump coronary artery bypass grafting in patients with multivessel coronary artery disease. J Thorac Cardiovasc Surg 2023; 166: 1446–1455. e4.
|
| [55] |
Lin S, Guan CD, Wu F, et al. Coronary artery bypass grafting and percutaneous coronary intervention in patients with chronic total occlusion and multivessel disease. Circ Cardiovasc Interv 2022; 15: e011312. doi: 10.1161/CIRCINTERVENTIONS.121.011312
|
| [56] |
Wang K, Wang L, Cong HL, et al. A comparison of drug-eluting stent and coronary artery bypass grafting in mildly to moderately ischemic heart failure. ESC Heart Fail 2022; 9: 1749−1755. doi: 10.1002/ehf2.13852
|
| [57] |
Zhao XY, Xu LJ, Jiang L, et al. Real-world outcomes of different treatment strategies in patients with diabetes and three-vessel coronary disease: a mean follow-up 6.3 years study from China. Cardiovasc Diabetol 2021; 20: 16. doi: 10.1186/s12933-020-01193-3
|
| [58] |
Fan Q, Liu J, Xu Y, et al. Real-world outcomes of revascularization strategies in patients with left ventricular dysfunction and three-vessel coronary disease stratified by mitral regurgitation. Front Cardiovasc Med 2021; 8: 675722. doi: 10.3389/fcvm.2021.675722
|
| [59] |
Qu JY, Zhang DW, Zhang H, et al. Preoperative clopidogrel and outcomes in patients with acute coronary syndrome undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg 2022; 163: 1044–1052. e15.
|
| [60] |
Wang ES, Yuan X, Wang Y, et al. Blood conservation outcomes and safety of tranexamic acid in coronary artery bypass graft surgery. Int J Cardiol 2022; 348: 50−56. doi: 10.1016/j.ijcard.2021.12.017
|
| [61] |
Li X, Gu DC, Wang XQ, et al. Trends of coronary artery bypass grafting performance in a cohort of hospitals in China between 2013 and 2018. Circ Cardiovasc Qual Outco mes 2021; 14: e007025. doi: 10.1161/CIRCOUTCOMES.120.007025
|
| [62] |
Zhang DW, Gu DC, Rao CF, et al. Outcome differences between surgeons performing first and subsequent coronary artery bypass grafting procedures in a day: a retrospective comparative cohort study. BMJ Qual Saf 2023; 32: 192−201. doi: 10.1136/bmjqs-2021-014244
|
| [63] |
Yang SC, Han YT, Yu CQ, et al. Development of a model to predict 10-year risk of ischemic and hemorrhagic stroke and ischemic heart disease using the China Kadoorie Biobank. Neurology 2022; 98: e2307−e2317. doi: 10.1212/WNL.98.18_supplement.2307
|
| [64] |
Lu XF, Liu ZY, Cui QM, et al. A polygenic risk score improves risk stratification of coronary artery disease: a large-scale prospective Chinese cohort study. Eur Heart J 2022; 43: 1702−1711. doi: 10.1093/eurheartj/ehac093
|
| [65] |
Zhao XY, Li JW, Liu FC, et al. The PRECISE-DAPT score and five-year outcomes after percutaneous coronary intervention: a large-scale, real-world study from China. Eur Heart J Qual Care Clin Outcomes 2022; 8: 812−820. doi: 10.1093/ehjqcco/qcab068
|
| [66] |
Xu W, Yang YM, Zhu J, et al. Predictive value of the stress hyperglycemia ratio in patients with acute ST-segment elevation myocardial infarction: insights from a multi-center observational study. Cardiovasc Diabetol 2022; 21: 48. doi: 10.1186/s12933-022-01479-8
|
| [67] |
Song L, Wang Y, Guan CD, et al. Impact of periprocedural myocardial injury and infarction definitions on long- term mortality after chronic total occlusion percutaneo us coronary intervention. Circ Cardiovasc Interv 2021; 14: e010923. doi: 10.1161/CIRCINTERVENTIONS.121.010923
|
| [68] |
Xiong SQ, Chen Q, Chen X, et al. Adjustment of the GRACE score by the triglyceride glucose index improves the prediction of clinical outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Diabetol 2022; 21: 145. doi: 10.1186/s12933-022-01582-w
|
| [69] |
Zhao Q, Zhang TY, Cheng YJ, et al. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc Diabetol 2020; 19: 108. doi: 10.1186/s12933-020-01086-5
|
| [70] |
Li Z, He YY, Wang S, et al. Association between triglyceride glucose index and carotid artery plaque in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabe tol 2022; 21: 38. doi: 10.1186/s12933-022-01470-3
|
| [71] |
Zhao YC, Lu XY, Wan F, et al. Disruption of circadian rhythms by shift work exacerbates reperfusion injury in myocardial infarction. J Am Coll Cardiol 2022; 79: 2097−2115. doi: 10.1016/j.jacc.2022.03.370
|
| [72] |
Gao JW, Hao QY, Lu LY, et al. Associations of long-term physical activity trajectories with coronary artery calcium progression and cardiovascular disease events: results from the CARDIA study. Br J Sports Med 2022; 56: 854−861. doi: 10.1136/bjsports-2021-105092
|
| [73] |
Li SD, Gao XJ, Yang JG, et al. Number of standard modifiable risk factors and mortality in patients with first-presentation ST-segment elevation myocardial infarction: insights from China Acute Myocardial Infarction registry. BMC Med 2022; 20: 217. doi: 10.1186/s12916-022-02418-w
|
| [74] |
Wang YN, Gao XQ, Zhao ZJ, et al. Predictive value of type D personality for cardiovascular events in young patients with acute myocardial infarction: a prospective, observational study. Eur J Prev Cardiol 2022; 29: e100−e101. doi: 10.1093/eurjpc/zwab030
|
| [75] |
Wang X, Zhao GQ, Zhou MG, et al. Trends in bleeding events among patients with acute coronary syndrome in China, 2015 to 2019: insights from the CCC-ACS project. Front Cardiovasc Med 2021; 8: 769165. doi: 10.3389/fcvm.2021.769165
|
| [76] |
Chen L, Tan YL, Yu CQ, et al. Educational disparities in ischaemic heart disease among 0.5 million Chinese adults: a cohort study. J Epidemiol Community Health 2021; 75: 1033−1043. doi: 10.1136/jech-2020-216314
|