| Citation: | Please cite this article as: Franke KB, Bhatia D, Roberts-Thomson RL, Psaltis PJ. Aortic valve replacement reduces mortality in moderate aortic stenosis: a systematic review and meta-analysis. J Geriatr Cardiol 2023; 20(1): 61−67. DOI: 10.26599/1671-5411.2023.01.003. |
Aortic stenosis (AS) has a high and increasing global burden, primarily driven by population aging, and is associated with significant mortality and morbidity. While aortic valve replacement (AVR) is primarily reserved for patients with severe symptomatic AS, recent population data have shown that patients with moderate AS have a similarly high 5-year mortality.[1] However, the role of early intervention in moderate AS remains unclear, with current guidelines recommending monitoring for disease progression and AVR (transcatheter or surgical) only if severe AS develops or if another indication for cardiac surgery is present.[2] We conducted this systematic review and meta-analysis to compare clinical outcomes between invasive (early AVR) and conservative (monitoring) management of moderate AS.
The English scientific literature was searched using Pubmed, Embase, and the Cochrane library up to 30th December 2021. Search strategy keywords included moderate aortic stenosis and aortic valve replacement ((“Moderate Aortic Stenosis” OR (“Moderate” [Title/Abstract] AND “Aortic Stenosis” [Title/Abstract]) AND (“Mortality” OR “Death” OR “MACE” OR “Major Adverse Cardiac Events” OR “Hospitalisation” OR “Outcomes”) AND (“Aortic Valve Replacement” OR “Transcatheter Aortic Valve Implantation” OR “Aortic Valve Surgery”)). Inclusion criteria for the search were studies that: (1) included patients with moderate AS, (2) who underwent either early AVR or (3) conservative management, and (4) compared all-cause mortality and outcomes between these two groups. Exclusion criteria included: (1) studies that were not primary clinical papers, (2) studies that did not specifically only include patients with moderate AS, (3) studies that did not report relevant clinical outcome data, and (4) studies where another study, from the same database with the same patient population, was already included. Study selection and data extraction were performed by two investigators independently (KBF and DB), using the inclusion and exclusion criteria. Reference lists of review articles were also evaluated for potential studies. Data between independent searches were then collated and checked for discrepancies. All included studies were also evaluated for bias using a modified Newcastle Ottawa Scale.
Statistical analysis was performed using Stata 15 (StataCorp, TX). All included studies compared mortality between groups with a cox proportional-hazard regression model with AVR as a time-dependent covariate; thus, these hazard ratios were meta-analysed and forest plots generated using the metan function. Random-effects meta-analysis was performed to determine effect estimates, allowing for heterogeneity between studies. Heterogeneity was assessed using the I2 statistic. Publication bias was assessed with visual assessment for symmetry of generated funnel plots and using the Egger test. Poisson distribution was used to generate incidence rates and confidence intervals. Meta-regression was then performed for incidence rates against variables of interest. Our study complies with the MOOSE group and PRISMA statements (PRISMA), and was registered with the PROSPERO International register for systematic reviews [CRD42021189396].
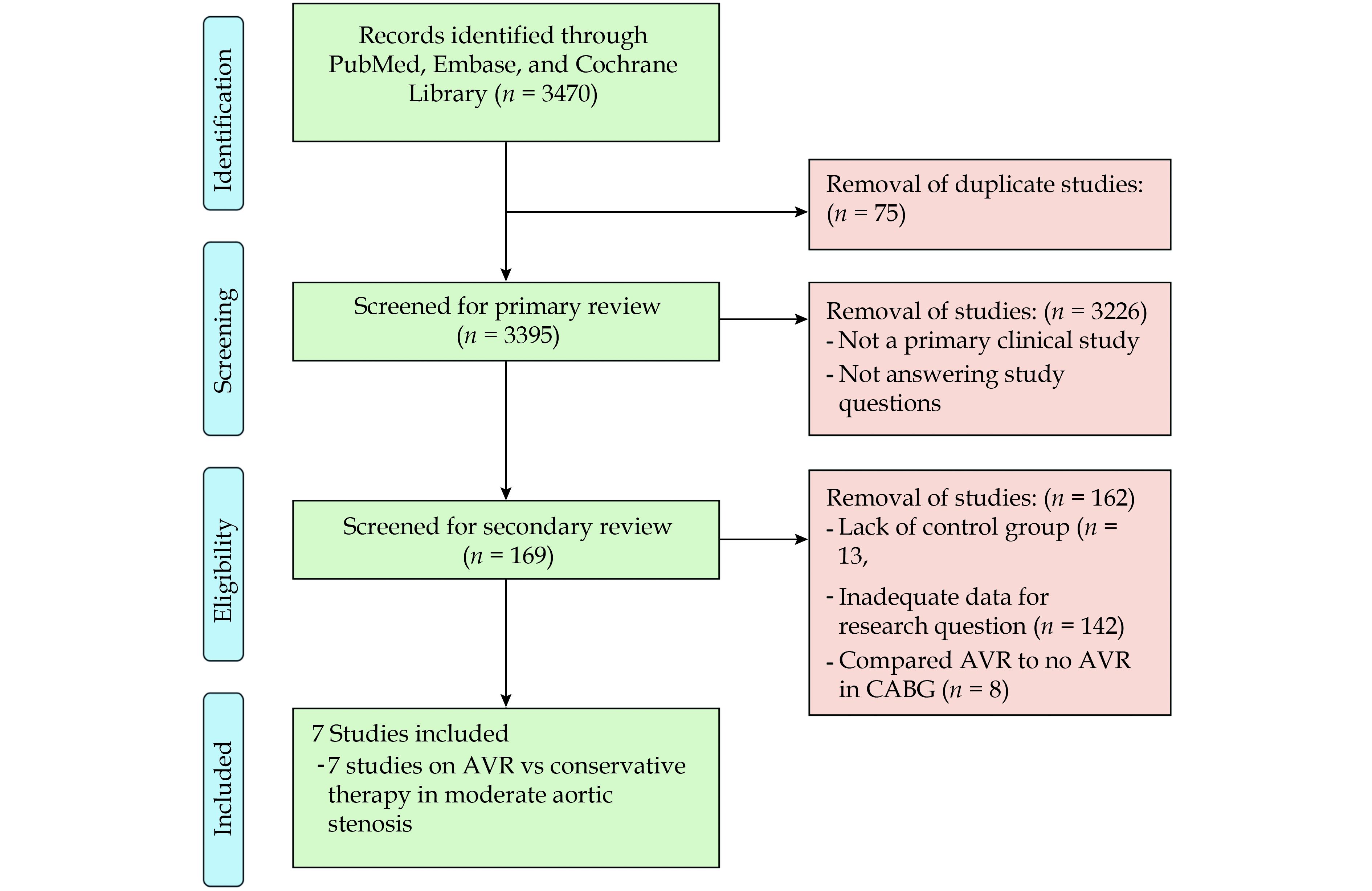
Our literature search yielded 3470 publications. Seventy five duplicates were excluded, leaving 3395 publications for a title and abstract review, after which 169 articles were eligible for full-text review. Full-text review excluded 162 of the remaining articles, leaving 7 observational studies, one of which was prospective, for inclusion in the final analysis (Figure 1). Overall, these 7 studies compared early AVR with conservative management in moderate AS,[3-9] totalling 4,827 patients. Study characteristics (Table 1) and patient characteristics (Table 2) are as shown. No study reported baseline patient characteristics in early AVR vs conservative management groups. Of the 4,827 included patients, 1,556 had an early intervention with AVR and 3,271 underwent conservative management. Median follow-up varied from 1.2 to 9.5 years across studies. Three studies exclusively included patients with left ventricular ejection fraction (LVEF) < 50% and one with LVEF > 50%. The three remaining studies included all ranges of LVEF. All studies treated AVR as a time-dependent co-variable in cox-regression multivariate analysis of all-cause mortality.
| Study (author, year) | Study design | Patient cohort | Recruitment strategy | Definition of moderate AS |
| Amanullah, 2021[6] | Retrospective, observational, single centre | Moderate aortic stenosis on echocardiogram | Retrospective review of echocardiograms from 2001-2018 | (1) AVA: 1.0-1.5 cm2 |
| Samad, 2016[5] | Retrospective, observational, echocardiogram database | LVEF < 50%, moderate aortic stenosis on echocardiogram | Retrospective review of echocardiograms from 1995-2014 | (1) AVA: 1.0-1.5 cm2 (2) Mean aortic gradient: 25-39 mmHg (3) Peak aortic jet velocity 3-4 m/s |
| Chew, 2021[9] | Retrospective, observational, single centre | Asymptomatic, LVEF > 50%, moderate aortic stenosis on echocardiogram | Retrospective review of echocardiograms from 2001-2020 | (1) Peak aortic velocity: 3.0-4.0 m/s (2) Mean aortic gradient: 20-40 mmHg (3) Aortic valve area: 1.0-1.5 cm2; (4) Indexed AVA: 0.60-0.85 cm2/ m2 (5) Velocity ratio: 0.25-0.50 |
| Du, 2021[8] | Prospective, observational, multi-centre | Moderate aortic stenosis on echocardiogram | Prospective enrolment of patients diagnosed with moderate AS on echocardiogram | (1) Peak aortic velocity: 3.0-4.0 m/s (2) Mean aortic gradient: 20- 40 mmHg (3) Aortic valve area: 1.0-1.5 cm2; (4) Indexed AVA: 0.60-0.85 cm2/ m2 (5) Dimensionless index: 0.25-0.50 |
| Delesalle, 2019[3] | Prospective, observational, multi-centre | LVEF > 50%, moderate AS on echocardiogram | Prospective enrolment of patients diagnosed with moderate AS on echocardiogram | (1) AVA: 1.0-1.5 cm2 |
| Jean, 2021[7] | Retrospective, observational, multi-centre | LVEF < 50%, moderate AS on echocardiogram | Retrospective review of echocardiograms from 2010-2015 | (1) AVA: 1.0-1.5 cm2 (2) Peak aortic jet velocity: 2-4 m/s at rest or after dobutamine stress echocardiography |
| Moon, 2020[4] | Retrospective, observational, echocardiogram database | LVEF < 50%, moderate AS on echocardiogram | Retrospective review of echocardiograms from 2001-2017 | (1) AVA: 1.0-1.5 cm2 (2) Maximal aortic valve velocity: 2-4 m/s |
| AS: aortic stenosis; AVA: aortic valve area; LVEF: left ventricular ejection fraction. | ||||
| Study (author, year) | Number of patients | Age | Male | CCF | EF | HTN | DM | AVR performed |
| Amanullah, 2021[6] | 1245 | 70.9 ± 12.3 | 622 (50.0%) | 418 (33.6%) | 58.3 ± 12.7 | 986 (79.2%) | 435 (34.9%) | 373 (30.0%) |
| Samad, 2016[5] | 1090 | 75 [67, 82] | 698 (64.0%) | 652 (59.8%) | NR | 717 (65.8%) | 392 (36.0%) | 287 (26.3%) |
| Chew, 2021[9] | 738 | 73.0 ± 12.0 | 439 (59.5%) | N/A | 64 ± 5 | 563 (76.3%) | 300 (40.7%) | 57 (7.7%) |
| Du, 2021[8] | 729 | 76 [67, 84] | 437 (59.9%) | N/A | 58 [45, 63] | 585 (80.2%) | 236 (32.4%) | 83 (11.4%) |
| Delesalle, 2019[3] | 508 | 75 ± 11 | 287 (56.5%) | 45 (8.9%) | 64 ± 8 | 398 (78.3%) | 246 (48.4%) | 113 (22.3%) |
| Jean, 2021[7] | 262 | 73.4 ± 10.4 | 203 (77.5%) | 262 (100%) | 38.5 ± 9.6 | 192 (73.3%) | 97 (37.0%) | 44 (16.8%) |
| Moon, 2020[4] | 255 | 70.1 ± 11.3 | 158 (62.0%) | 255 (100%) | 40.3 ± 8.5 | 150 (58.8%) | 89 (34.9%) | 37 (14.5%) |
| Data are presented as mean ± SD, median [IQR], or n (%). AVR: aortic valve replacement; CCF: congestive cardiac failure; DM: diabetes mellitus; EF: ejection fraction; HTN: hypertension; NR: not reported. | ||||||||
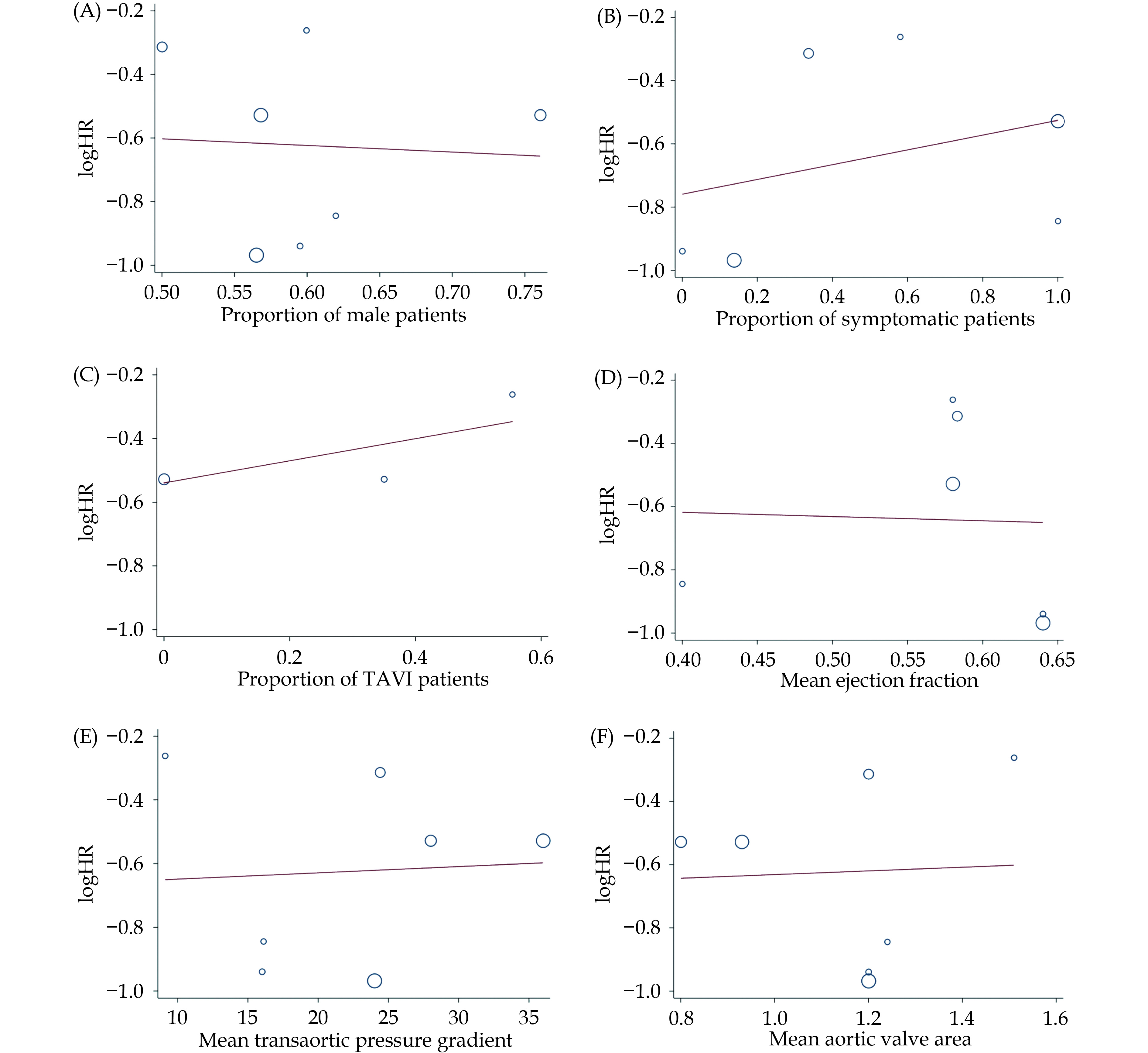
Intervention with surgical or transcatheter AVR was associated with a 45% decreased risk of all-cause mortality (HR = 0.55 [0.42-0.68], I2 = 51.5%, P < 0.001) (Figure 2). Meta-regression of hazard ratios across studies revealed no significant association of treatment effect with the type of surgical intervention (transcatheter vs. surgical), gender, aortic valve area or pressure gradient, ejection fraction, or presence of symptoms (Figure 3). All studies were representative of the overall cohort with appropriate sample sizes. Funnel plots were symmetrical and Egger values were not significant for publication bias. Modified Newcastle Ottawa scores revealed there was a low risk of detection and information biases in all studies.
Our study shows that there is already considerable observational data to indicate that early AVR for moderate AS may result in lower all-cause mortality. Historically, AVR has been reserved for symptomatic patients with severe AS. Recently, the AVA TAR trial demonstrated a mortality benefit from surgical AVR in severe AS even in the absence of symptoms and reduced left ventricular fraction.[10] Furthermore, as the natural history of moderate AS has been studied, it has become increasingly clear that its course is not benign.[11] While the available data in moderate AS is currently limited to small observational cohorts, the recent literature suggests that earlier intervention may be beneficial.[12] A histological study by Hein, et al.[13] demonstrated that myocardial changes in severe AS are self-perpetuating, even after AVR. This implies that intervention in the later stages of AS may not be able to reverse myocyte damage and fibrosis, and pathological processes in the myocardium may continue even if AVR is performed. Similarly, Chin, et al.[14] a used cardiac magnetic resonance imaging to show that all-cause mortality in patients with severe AS relates to the extent of myocardial fibrosis. Interestingly, neither the presence of symptoms nor LVEF was able to predict fibrosis, demonstrating that this process often occurs before these later stage markers of progression are present.
Recently, a study by Ito, et al.[15] of 696 patients with moderate AS demonstrated a 40% mortality rate over a median 3.4-year follow-up period, equating to a 2.4-fold increased risk compared to age and sex-matched mortality in the general population. Increased mortality was associated with an LVEF < 50% and the presence of symptoms, reflecting current guideline criteria for AVR in severe AS, but not moderate AS. However, once these clinical features are present, there is often already irreversible left ventricular myocyte damage and fibrosis.[14] Therefore, earlier intervention with AVR, even in patients with preserved LVEF and minimal symptoms may alter this natural history, preventing irreversible myocardial damage and improving long-term mortality. This is consistent with our meta-analyzed results which highlight that the mortality benefit from early intervention in moderate AS may not just be limited to higher-risk patients with reduced systolic function and/or symptoms.
Despite this being the first meta-analysis to demonstrate a mortality improvement in moderate AS patients undergoing early AVR, there are some limitations to our study. We were restricted in our ability to analyze baseline clinical characteristics and unable to evaluate other outcomes besides all-cause mortality, because of a lack of available data in the included studies. Furthermore, none of these studies randomised patients to AVR or conservative treatment at baseline; rather, by using AVR as a time-dependent covariable, hazard ratios were derived from patients that underwent AVR at some point in their follow-up, introducing the potential for selection bias of patients appropriate for AVR. Unfortunately, due to limitations in data reporting and statistical analysis, a subgroup analysis of those with impaired LVEF could not be performed. Similarly, there was only limited use of transcatheter aortic valve replacement (TAVR) and no published data specifically comparing TAVR or surgical AVR (SAVR) to conservative treatment in patients with moderate AS. Furthermore, only two studies specified proportions of TAVR and SAVR patients in the intervention group, and only one study specified that patients underwent SAVR alone. All other studies did not report proportions of patients undergoing TAVR or SAVR. Although we performed a meta-regression analysis of the type of intervention performed in these three studies, it was negative and largely uninterpretable due to underpowering. Therefore, while TAVR provides a promising intervention for early AVR in appropriate patients, information is now needed to determine its longevity, complications and benefits in AS of moderate severity.
Furthermore, our meta-analysis did not explore the utility of AVR in mixed aortic valve disease (MAVD), where both AS and aortic regurgitation are present. It has been shown that MAVD progresses at a more rapid rate than isolated aortic stenosis or regurgitation alone. The increased pressure and volume load from aortic stenosis and regurgitation, respectively, may accelerate myocardial fibrosis and irreversible myocyte dysfunction at a faster rate than isolated AS11. Thus, the ideal timing of surgery in these patients, although not yet fully explored, may indeed be earlier in MAVD.
Lastly, the implications of an early AVR strategy are broad. Mortality benefits must be balanced against the risks of intervention: the durability of bioprosthetic valves, risks from anticoagulation and risk of infective endocarditis are all inherent risks that may be increased if an early intervention strategy is adopted. In light of these limitations, although these results show promise for the use of AVR in moderate AS, possible complications of early intervention have not been explored thoroughly in this meta-analysis, and prospective randomized control trials are needed to confirm its findings and rigorously explore outcomes.
This meta-analysis consolidates the observational literature highlighting that early AVR may be beneficial for patients with moderate AS before they reach the criteria recommended by current guidelines. However, prospective randomized trials, such as the upcoming PROGRESS study (NCT 04889872) and the TAVI-unload trial (NCT 02661451), are required to verify our findings and further explore the utility of early intervention, particularly with TAVR.
| [1] |
Strange G, Stewart S, Celermajer D, et al. Poor long-term survival in patients with moderate aortic stenosis. J Am Coll Cardiol 2019; 74: 1851−1863. doi: 10.1016/j.jacc.2019.08.004
|
| [2] |
Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021; 143: e35−e71.
|
| [3] |
Delesalle G, Bohbot Y, Rusinaru D, et al. Characteristics and prognosis of patients with moderate aortic stenosis and preserved left ventricular ejection fraction. J Am Heart Assoc 2019; 8: e011036. doi: 10.1161/JAHA.118.011036
|
| [4] |
Moon I, Kim M, Choi JW, et al. Early surgery versus watchful waiting in patients with moderate aortic stenosis and left ventricular systolic dysfunction. Korean Circ J 2020; 50: 791−800. doi: 10.4070/kcj.2020.0037
|
| [5] |
Samad Z, Vora AN, Dunning A, et al. Aortic valve surgery and survival in patients with moderate or severe aortic stenosis and left ventricular dysfunction. Eur Heart J 2016; 37: 2276−2286. doi: 10.1093/eurheartj/ehv701
|
| [6] |
Amanullah MR, Pio SM, Ng ACT, et al. Prognostic implications of associated cardiac abnormalities detected on echocardiography in patients with moderate aortic stenosis. JACC Cardiovasc Imaging 2021; 14: 1724−1737. doi: 10.1016/j.jcmg.2021.04.009
|
| [7] |
Jean G, Van Mieghem NM, Gegenava T, et al. Moderate aortic stenosis in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 2021; 77: 2796−2803.
|
| [8] |
Du Y, Gössl M, Garcia S, et al. Natural history observations in moderate aortic stenosis. BMC Cardiovasc Disord 2021; 21: 108. doi: 10.1186/s12872-021-01901-1
|
| [9] |
Chew NW, Kong G, Ngiam JN, et al. Comparison of outcomes of asymptomatic moderate aortic stenosis with preserved left ventricular ejection fraction in patients ≥ 80 years versus 70-79 years versus < 70 years. Am J Cardiol 2021; 157: 93−100. doi: 10.1016/j.amjcard.2021.07.015
|
| [10] |
Banovic M, Putnik S, Penicka M, et al. Aortic valve replacement versus conservative treatment in asymptomatic severe aortic stenosis: The AVATAR Trial. Circulation 2022; 145: 648−658. doi: 10.1161/CIRCULATIONAHA.121.057639
|
| [11] |
Koerber JP, Bennetts JS, Psaltis PJ. Early valve replacement for severe aortic valve disease: effect on mortality and clinical ramifications. J Clin Med 2020; 9: 2694. doi: 10.3390/jcm9092694
|
| [12] |
Everett RJ, Clavel MA, Pibarot P, Dweck MR. Timing of intervention in aortic stenosis: a review of current and future strategies. Heart 2018; 104: 2067−2076. doi: 10.1136/heartjnl-2017-312304
|
| [13] |
Hein S, Arnon E, Kostin S, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation 2003; 107: 984−991. doi: 10.1161/01.CIR.0000051865.66123.B7
|
| [14] |
Chin CWL, Everett RJ, Kwiecinski J, et al. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging 2017; 10: 1320−1333. doi: 10.1016/j.jcmg.2016.10.007
|
| [15] |
Ito S, Miranda WR, Nkomo VT, et al. Prognostic risk stratification of patients with moderate aortic stenosis. J Am Soc Echocardiogr 2021; 34: 248−256. doi: 10.1016/j.echo.2020.10.012
|
| Study (author, year) | Study design | Patient cohort | Recruitment strategy | Definition of moderate AS |
| Amanullah, 2021[6] | Retrospective, observational, single centre | Moderate aortic stenosis on echocardiogram | Retrospective review of echocardiograms from 2001-2018 | (1) AVA: 1.0-1.5 cm2 |
| Samad, 2016[5] | Retrospective, observational, echocardiogram database | LVEF < 50%, moderate aortic stenosis on echocardiogram | Retrospective review of echocardiograms from 1995-2014 | (1) AVA: 1.0-1.5 cm2 (2) Mean aortic gradient: 25-39 mmHg (3) Peak aortic jet velocity 3-4 m/s |
| Chew, 2021[9] | Retrospective, observational, single centre | Asymptomatic, LVEF > 50%, moderate aortic stenosis on echocardiogram | Retrospective review of echocardiograms from 2001-2020 | (1) Peak aortic velocity: 3.0-4.0 m/s (2) Mean aortic gradient: 20-40 mmHg (3) Aortic valve area: 1.0-1.5 cm2; (4) Indexed AVA: 0.60-0.85 cm2/ m2 (5) Velocity ratio: 0.25-0.50 |
| Du, 2021[8] | Prospective, observational, multi-centre | Moderate aortic stenosis on echocardiogram | Prospective enrolment of patients diagnosed with moderate AS on echocardiogram | (1) Peak aortic velocity: 3.0-4.0 m/s (2) Mean aortic gradient: 20- 40 mmHg (3) Aortic valve area: 1.0-1.5 cm2; (4) Indexed AVA: 0.60-0.85 cm2/ m2 (5) Dimensionless index: 0.25-0.50 |
| Delesalle, 2019[3] | Prospective, observational, multi-centre | LVEF > 50%, moderate AS on echocardiogram | Prospective enrolment of patients diagnosed with moderate AS on echocardiogram | (1) AVA: 1.0-1.5 cm2 |
| Jean, 2021[7] | Retrospective, observational, multi-centre | LVEF < 50%, moderate AS on echocardiogram | Retrospective review of echocardiograms from 2010-2015 | (1) AVA: 1.0-1.5 cm2 (2) Peak aortic jet velocity: 2-4 m/s at rest or after dobutamine stress echocardiography |
| Moon, 2020[4] | Retrospective, observational, echocardiogram database | LVEF < 50%, moderate AS on echocardiogram | Retrospective review of echocardiograms from 2001-2017 | (1) AVA: 1.0-1.5 cm2 (2) Maximal aortic valve velocity: 2-4 m/s |
| AS: aortic stenosis; AVA: aortic valve area; LVEF: left ventricular ejection fraction. | ||||
| Study (author, year) | Number of patients | Age | Male | CCF | EF | HTN | DM | AVR performed |
| Amanullah, 2021[6] | 1245 | 70.9 ± 12.3 | 622 (50.0%) | 418 (33.6%) | 58.3 ± 12.7 | 986 (79.2%) | 435 (34.9%) | 373 (30.0%) |
| Samad, 2016[5] | 1090 | 75 [67, 82] | 698 (64.0%) | 652 (59.8%) | NR | 717 (65.8%) | 392 (36.0%) | 287 (26.3%) |
| Chew, 2021[9] | 738 | 73.0 ± 12.0 | 439 (59.5%) | N/A | 64 ± 5 | 563 (76.3%) | 300 (40.7%) | 57 (7.7%) |
| Du, 2021[8] | 729 | 76 [67, 84] | 437 (59.9%) | N/A | 58 [45, 63] | 585 (80.2%) | 236 (32.4%) | 83 (11.4%) |
| Delesalle, 2019[3] | 508 | 75 ± 11 | 287 (56.5%) | 45 (8.9%) | 64 ± 8 | 398 (78.3%) | 246 (48.4%) | 113 (22.3%) |
| Jean, 2021[7] | 262 | 73.4 ± 10.4 | 203 (77.5%) | 262 (100%) | 38.5 ± 9.6 | 192 (73.3%) | 97 (37.0%) | 44 (16.8%) |
| Moon, 2020[4] | 255 | 70.1 ± 11.3 | 158 (62.0%) | 255 (100%) | 40.3 ± 8.5 | 150 (58.8%) | 89 (34.9%) | 37 (14.5%) |
| Data are presented as mean ± SD, median [IQR], or n (%). AVR: aortic valve replacement; CCF: congestive cardiac failure; DM: diabetes mellitus; EF: ejection fraction; HTN: hypertension; NR: not reported. | ||||||||