| Citation: | Please cite this article as: SHANG Z, ZHAO ML, WANG XY, GAO W. Multidisciplinary team management and long-term prognosis of an elderly patient with severe cardiac amyloidosis complicated with multiple myeloma. J Geriatr Cardiol 2022; 19(5): 398−402. DOI: 10.11909/j.issn.1671-5411.2022.05.006. |
Immunoglobulin light chain amyloidosis (AL) is one of the most frequent causes of cardiac amyloidosis (CA),[1] with an estimated prevalence of 8 to 12 per million.[2-4] Multiple myeloma (MM) is hematological neoplasia originating from plasma cells, which is the most common disease that can lead to CA. The median age of patients with MM at diagnosis is about 65 years old. In this age group, cardiovascular diseases often co-exist, increasing the risk of adverse events related to MM treatment. By convention, the prognosis of AL-CA with MM is extremely poor, with a median survival time of five months.[5] The degree of cardiac involvement has a decisive impact on the prognosis of AL-CA patients.[6] Transmural pattern indicating diffuse myocardial involvement diagnosed by late gadolinium enhancement (LGE) represents the worst prognosis (hazard ratio for death compared with patients without transmural LGE: 5.4; 95% CI: 2.1–13.7; P < 0.001).[6]
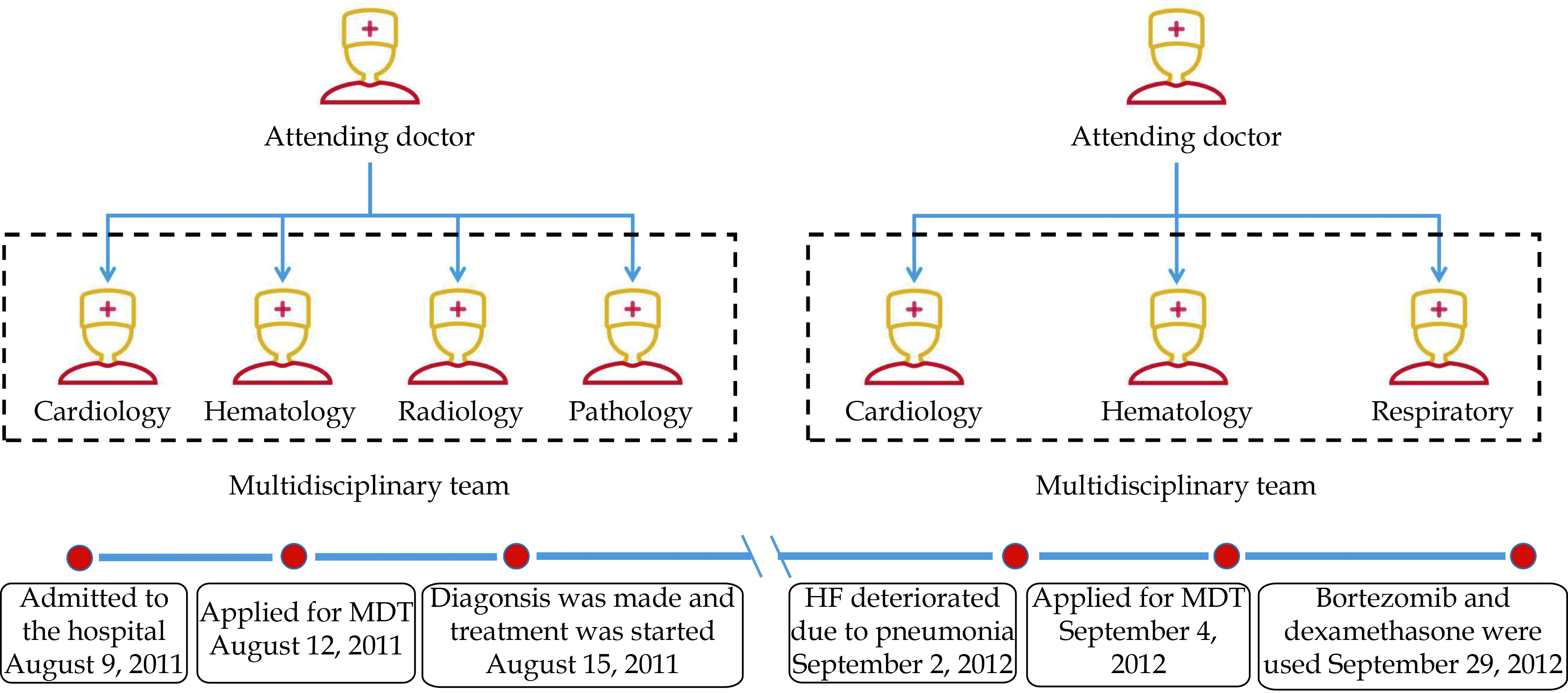
An important reason for the high mortality of patients with CA is that the diagnosis process involves many disciplines, including cardiology, hematology, radiology, pathology, and other departments, which may cause a delay in diagnosis. Moreover, the patients’ condition is complex, which means there may be changes in the condition at any time during the follow-up process. This requires close follow-up by multiple departments. A multidisciplinary team (MDT) approach in the management of heart failure (HF) is a key recommendation in recent guidelines, receiving a Class IA recommendation in the European Society of Cardiology guidelines to reduce mortality and HF hospitalization and a Class IB recommendation in the American College of Cardiology Foundation/American Heart Association guidelines.[7,8] Compared with MDT, traditional expert consultation, which is a disease management model widely used in China, has no standardized team structure and is characterized by randomness and temporary.
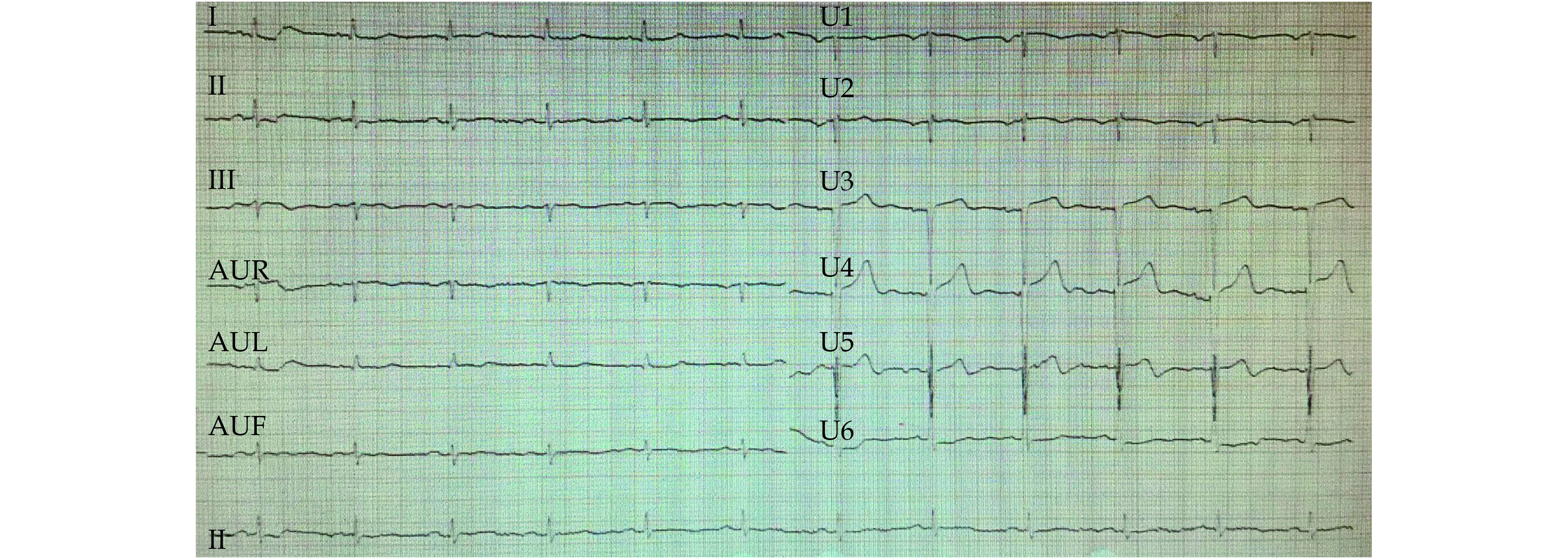
In our clinical practice, we treated a 66-year-old elderly patient with severe AL-CA complicated with MM who obtained a long-term survival for more than ten years after receiving MDT evaluation and long-term follow-up. This patient was a 66-year-old male who was referred to the department of cardiology, the Third Hospital of Peking University on August 9, 2011, with a major complaint of exertional shortness of breath, fatigue, and edema of lower extremities during the last month. No clinically significant history was provided other than eight years of grade 2 hypertension which was well controlled without any meditation. The patient denied any contact with bio-physic-chemical hazards. Physical examination showed normal rhythm and blood pressure, clear lung field, left-sided enlargement of heart boundary, loud P2, 3/6 systolic murmur in the mitral acoustic zone, jugular vein extension, hepatomegaly, and slight pitting edema in lower extremities. Relevant laboratory tests revealed mild anemia (hemoglobin: 118 g/L), elevated N-terminal pro-B-type natriuretic peptide (NT-pro BNP) (2156 pg/mL, normal < 125 pg/mL) and cardiac troponin T (0.11 ng/mL, normal < 0.1 ng/mL), normal liver enzymes, serum creatinine, total protein and albumin. A two-dimensional echocardiogram revealed marked symmetrical thickening of the left ventricular (LV) wall with sparkled appearance, enlargement of left atrium, mitral and tricuspid valve regurgitation, compromised left ventricular ejection fraction (LVEF, 40%), and decreased LV diastolic function (E/Em = 18). Chest X-ray revealed cardiomegaly (cardiothoracic ratio of 0.6). Electrocardiogram showed a normal sinus rhythm, low voltage of limb leads, and non-specific T-wave changes (Figure 1).
Since the diagnosis of HF was clear, with New York Heart Association (NYHA) cardiac function be level III, we further developed measurements to determine the etiology of HF. Coronary angiography showed no stenosis of main coronary arteries, excluding ischemic cardiomyopathy. Since the manifestations of echocardiogram and electrocardiogram (ECG) were very special (low voltage in limb leads in ECG and diffuse LV hypertrophy in echocardiography), along with other clinical findings, we speculated that the patient was likely to have CA and excluded other common causes of HF (e.g., hypertensive cardiomyopathy, hypertrophic cardiomyopathy, and dilated cardiomyopathy). In order to clarify the diagnosis and comprehensively evaluate the patient’s condition, we organized the MDT approach, including cardiology, hematology, radiology, and pathology (Figure 2). The MDT group finally decided to conduct hematology examinations, cardiac magnetic resonance (CMR), myocardial biopsy, and bone marrow puncture, to clarify diagnosis and disease classification as soon as possible on the basis of improving HF, and evaluate the prognosis of patients to formulate the final treatment plan and strive to improve the long-term prognosis of the patient. The results were as follows. The levels of λ light chain of both serum (1380 mg/dL, normal: 269-638 mg/dL) and urine (1280 mg/dL, normal: 0–5 mg/dL) were significantly elevated, while the level of κ light chain was normal. And serum electrophoresis showed a monoclonal band of IgG and duo clonal band of λ light chain, while CMR showed diffuse LGE in the LV wall (Figure 3), raising the suspicion of the existence of CA. Ultimately, we performed an endomyocardial biopsy which showed that specimens with deposition of eosinophilic amorphous extracellular material appeared pink-orange with standard light microscopy after staining with Congo red (Figure 4). According to the examination results, the diagnosis of AL-CA was clear. We further performed a bone marrow examination, finding that there were more than 30% premature plasma cells, which provided solid evidence for the diagnosis of MM. No other organ involvement was found.
The patient was initially treated with ramipril, torasemide, and spironolactone, which led to the improvement of dyspnea and edema, resulting in the recovery of NYHA cardiac function to level III, and a decrease of circulating NT-pro BNP level from 2156 to 1563 pg/mL. Importantly, the patient’s cardiac function has been well improved during the examination. After the findings of bone marrow examination and the endomyocardial biopsy came out, a prognosis evaluation and a treatment decision by the MDT group were established. Since the expected median survival time was only 14 months using Mayo Clinic Staging System (NT-Pro BNP > 1800 ng/L, cTnT > 0.025 μg/L, Free Light Chain [FLC]-diff < 180 mg/L, Mayo stage III),[9,10] and diffuse LGE pattern of CMR indicated severe cardiac involvement; the hematologist firstly decided to use thalidomide and one course of VD regimen (Velcade and dexamethasone). However, the patient refused to use Velcade for economic reasons. Therefore, the MDT group decided to use DT regimen (dexamethasone and thalidomide). After then, thalidomide (100 mg once a day) was used for long-term maintenance. The NYHA cardiac function was maintained at level II until one year later (September 2, 2012), and it deteriorated to level IV because the patient developed acute HF due to pneumonia. Echocardiography showed that LVEF was decreased to 35%, although serum λ light chain reduced to 916 mg/dL (Table 1). Once more, we conducted the MDT approach, including cardiology, respiratory, and hematology, to make the optimal treatment method. After the antibiotic therapy and improving HF treatment, the symptoms of the patient improved, with NYHA cardiac function recovered to level III. According to the patient’s condition, the MDT group decided to use VD regimen (Velcade: 1.75 mg d1, d4, d8, d11; and dexamethasone: 20 mg d1-4, d9-12) with the consent of the patient’s family. In the course of treatment, the cardiac function deteriorated again, with the NYHA cardiac function reduced to level IV. After active treatment, the symptoms of HF significantly improved, and chemotherapy was completed successfully. The patient continued to use thalidomide (100 mg once a day) for long-term maintenance treatment, with NYHA cardiac function gradually recovered to level III. At a two-year follow-up, the serum λ light chain had returned to normal (632 mg/dL) while the monoclonal band in the electrophoresis test was also eliminated. Echocardiography showed that the LVEF recovered to 58% but without a reduction in LV wall thickness, while the LV diastolic function had returned to normal (E/Em = 10). After discharge, the patient was regularly followed up in the cardiology and hematology outpatient department. During the very long-term follow-up of 10 years (last follow-up time: September 20, 2021, with the age of 76), the patient was free of cardiac strikes and remained in NYHA cardiac function of level II, and very satisfied with our treatment.
| Baseline (2011-08) | 1-year (2012-09) | 2-year (2013-09) | |
| NYHA level | III | IV | II |
| NT-pro BNP, < 125 pg/mL | 2156 | 27597 | 14341 |
| cTnT (ng/mL, < 0.1) | 0.11 | 0.117 | 0.102 |
| λ- Light chain (mg/dL, 269-638) | 1386 | 916 | 632 |
| κ-light chain (mg/dL, 574-1276) | 418 | 830 | 830 |
| FLC-diff | 96.8 | 8.6 | −19.8 |
| LVEF, % | 40 | 35 | 58 |
| LVEDD, mm | 52.6 | 54.4 | 52.5 |
| Septal thickness, mm | 12.8 | 13.5 | 13.4 |
| Posterior wall thickness, mm | 13 | 13.7 | 13.8 |
| RV anteroposterior diameter, mm | 26.7 | 26.2 | 24.7 |
| Em, cm/s | 6 | 6 | 6 |
| E/Em | 18 | 18 | 10 |
| cTnT: cardiac troponin T; FLC: free light chain; LVEDD: left ventricular end diastolic diameter; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; RV: right ventricular. | |||
This particular case report provides insights into the management of patients with AL-CA. The MDT approach is a diagnosis and treatment mode in which multidisciplinary experts discuss the case of a certain or a systematic disease and formulate the optimal treatment scheme for patients based on integrating the opinions of various disciplines. In the MDT approach, experts of various disciplines can comprehensively analyze the patient’s condition at the first time, and specify the most reasonable treatment scheme for the patient, so as to avoid misdiagnosis and improve medical efficiency and medical quality, which is especially suitable for the diagnosis and treatment of patients with HF. In our clinical practice, the patient underwent the MDT approach immediately after completing the preliminary assessment. As a result, it took only six days from admission to diagnosis and formulation of treatment strategy, which provided the possibility for patients to achieve long-term survival. During the first treatment period, the MDT group recommended the patient to use a treatment regimen containing bortezomib, who refused for economic reasons. Then, the MDT group decided to use the treatment scheme containing thalidomide under the condition of fully considering the patient’s own condition. At a one-year follow-up, the patient developed an exacerbation of HF due to pneumonia. After admission, the MDT group, including cardiology, hematology, and respiratory, was quickly started. After treatment of antibiotics and improving HF, the MDT group decided to use bortezomib for treatment after obtaining the consent of the patient. After discharge, the patient was regularly followed up in the cardiology and hematology outpatient department. In the treatment process of the patient, the MDT group provided timely and individualized treatment, combined with the patient’s own situation, providing strong support for very long-term survival.
Radial LV function is preserved in most patients with CA, which means that the LVEF is preserved or only mildly reduced.[11] However, most patients with CA show impaired diastolic function,[12] which can be emerged prior to the thickening of right ventricular or LV walls.[13,14] The primary determinant of prognosis in AL-CA was the extent of cardiac involvement,[3] which was divided into three types, including no LGE (34%), subendocardial LGE (39%), and transmural LGE (27%).[6] Patients with transmural LGE had a worse prognosis than patients without transmural LGE.[6] In this case, the CMR showed transmural LGE with significant thickening of the LV wall. Moreover, a two-year follow-up demonstrated that LV wall thickness did not decrease significantly, while LV systolic and diastolic functions were significantly improved, indicating that although early treatment could not cause changes in cardiac macro-structure, it could lead to a significant improvement in cardiac function.
This work was supported by grants from the National Natural Science Foundation of China (grant No. 81972149, 81871850), Beijing Natural Science Foundation (grant No. 7212125).
None
Zhi Shang and Menglin Zhao analyzed and interpreted the patient data and wrote the manuscript. Xinyu Wang and Wei Gao were major contributors in writing and revising the manuscript. All authors read and approved the final manuscript.
| [1] |
Maurer MS, Elliott P, Comenzo R, et al. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation 2017; 135: 1357−1377. doi: 10.1161/CIRCULATIONAHA.116.024438
|
| [2] |
Kyle RA, Linos A, Beard CM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood 1992; 79: 1817−1822.
|
| [3] |
Hemminki K, Li X, Försti A, et al. Incidence and survival in non-hereditary amyloidosis in Sweden. BMC Public Health 2012; 12: 974. doi: 10.1186/1471-2458-12-974
|
| [4] |
Pinney JH, Smith CJ, Taube JB, et al. Systemic amyloidosis in England: an epidemiological study. Br J Haematol 2013; 161: 525−532. doi: 10.1111/bjh.12286
|
| [5] |
Grogan M, Dispenzieri A, Gertz MA. Light-chain cardiac amyloidosis: strategies to promote early diagnosis and cardiac response. Heart 2017; 103: 1065−1072. doi: 10.1136/heartjnl-2016-310704
|
| [6] |
Fontana M, Pica S, Reant P, et al. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation 2015; 132: 1570−1579. doi: 10.1161/CIRCULATIONAHA.115.016567
|
| [7] |
McDonagh TA, Metra M, Adamo M, et al; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599−3726. doi: 10.1093/eurheartj/ehab368
|
| [8] |
Yancy CW, Jessup M, Bozkurt B, et al. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: e147−239. doi: 10.1016/j.jacc.2013.05.019
|
| [9] |
Kumar S, Dispenzieri A, Lacy MQ, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol 2012; 30: 989−995. doi: 10.1200/JCO.2011.38.5724
|
| [10] |
Kumar SK, Gertz MA, Dispenzieri A. Validation of Mayo clinic staging system for light chain amyloidosis with high-sensitivity troponin. J Clin Oncol 2019; 37: 171−173.
|
| [11] |
Bonderman D, Pölzl G, Ablasser K, et al. Diagnosis and treatment of cardiac amyloidosis: an interdisciplinary consensus statement. Wien Klin Wochenschr 2020; 132: 742−761. doi: 10.1007/s00508-020-01781-z
|
| [12] |
Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321−1360. doi: 10.1093/ehjci/jew082
|
| [13] |
Modesto KM, Dispenzieri A, Cauduro SA, et al. Left atrial myopathy in cardiac amyloidosis: implications of novel echocardiographic techniques. Eur Heart J 2005; 26: 173−179. doi: 10.1093/eurheartj/ehi040
|
| [14] |
Cappelli F, Porciani MC, Bergesio F, et al. Right ventricular function in AL amyloidosis: characteristics and prognostic implication. Eur Heart J Cardiovasc Imaging 2012; 13: 416−422. doi: 10.1093/ejechocard/jer289
|
| Baseline (2011-08) | 1-year (2012-09) | 2-year (2013-09) | |
| NYHA level | III | IV | II |
| NT-pro BNP, < 125 pg/mL | 2156 | 27597 | 14341 |
| cTnT (ng/mL, < 0.1) | 0.11 | 0.117 | 0.102 |
| λ- Light chain (mg/dL, 269-638) | 1386 | 916 | 632 |
| κ-light chain (mg/dL, 574-1276) | 418 | 830 | 830 |
| FLC-diff | 96.8 | 8.6 | −19.8 |
| LVEF, % | 40 | 35 | 58 |
| LVEDD, mm | 52.6 | 54.4 | 52.5 |
| Septal thickness, mm | 12.8 | 13.5 | 13.4 |
| Posterior wall thickness, mm | 13 | 13.7 | 13.8 |
| RV anteroposterior diameter, mm | 26.7 | 26.2 | 24.7 |
| Em, cm/s | 6 | 6 | 6 |
| E/Em | 18 | 18 | 10 |
| cTnT: cardiac troponin T; FLC: free light chain; LVEDD: left ventricular end diastolic diameter; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; RV: right ventricular. | |||