| Citation: | Please cite this article as: Christopher O, XU XF, LIN YF, ZHANG SZ, HUANG YQ, ZHONG XB, XIONG ZY, CHEN TSR, XU CG, ZHUANG XD, LIAO XX. Association between serum cystatin C level and hemodynamically significant aortic stenosis: a prospective cohort study. J Geriatr Cardiol 2021; 18(12): 986−995. DOI: 10.11909/j.issn.1671-5411.2021.12.003. |
Aortic stenosis (AS) is the most common aetiology of aortic valve disease (AVD), affecting 3% of people > 65 years of age and has a high prevalence among developed countries.[1–3] AS has been strongly related to adverse outcomes and an increased risk of cardiovascular morbidity.[1] Despite the limitation in prognosis, aortic valve replacement treatment remains its first-line treatment option for elderly and high-risk patients.[4,5] Various coronary heart disease (CHD) and chronic kidney disease (CKD) risk factors are reported to show a strong correlation with AS incidence.[2,6,7] Cystatin C (CysC) is a cysteine protease inhibitor involved in catabolism and plays an essential role in human vascular pathophysiology.[8] In addition, it is produced by all nucleated cells and eliminated from the bloodstream by glomerular filtration.[8] Circulating CysC levels have been proven an alternative surrogate parameter of renal dysfunction.[9,10] It is suggested as a more sensitive renal impairment marker, particularly in subjects with creatinine levels within normal limits. Similarly, CysC is a pro-inflammatory biomarker essential for the prognosis of coronary artery calcification and adverse CHD outcome in elderly patients.[8,11,12]
However, research is still needed for clarifying the association between AVD, AS and elevated serum CysC levels among individuals at high-risk in the general population. Therefore, the purpose of this study was to test the hypothesis that an increase in serum CysC levels would increase the risk of AS independent of other traditional risk factors in the Atherosclerosis Risk in Communities (ARIC) cohort, a community-based study of cardiovascular disease in the United States.
ARIC Institutional Review Boards approved the study protocol (NCT00005131) at all Fifield centres, and all participants provided written informed consent.
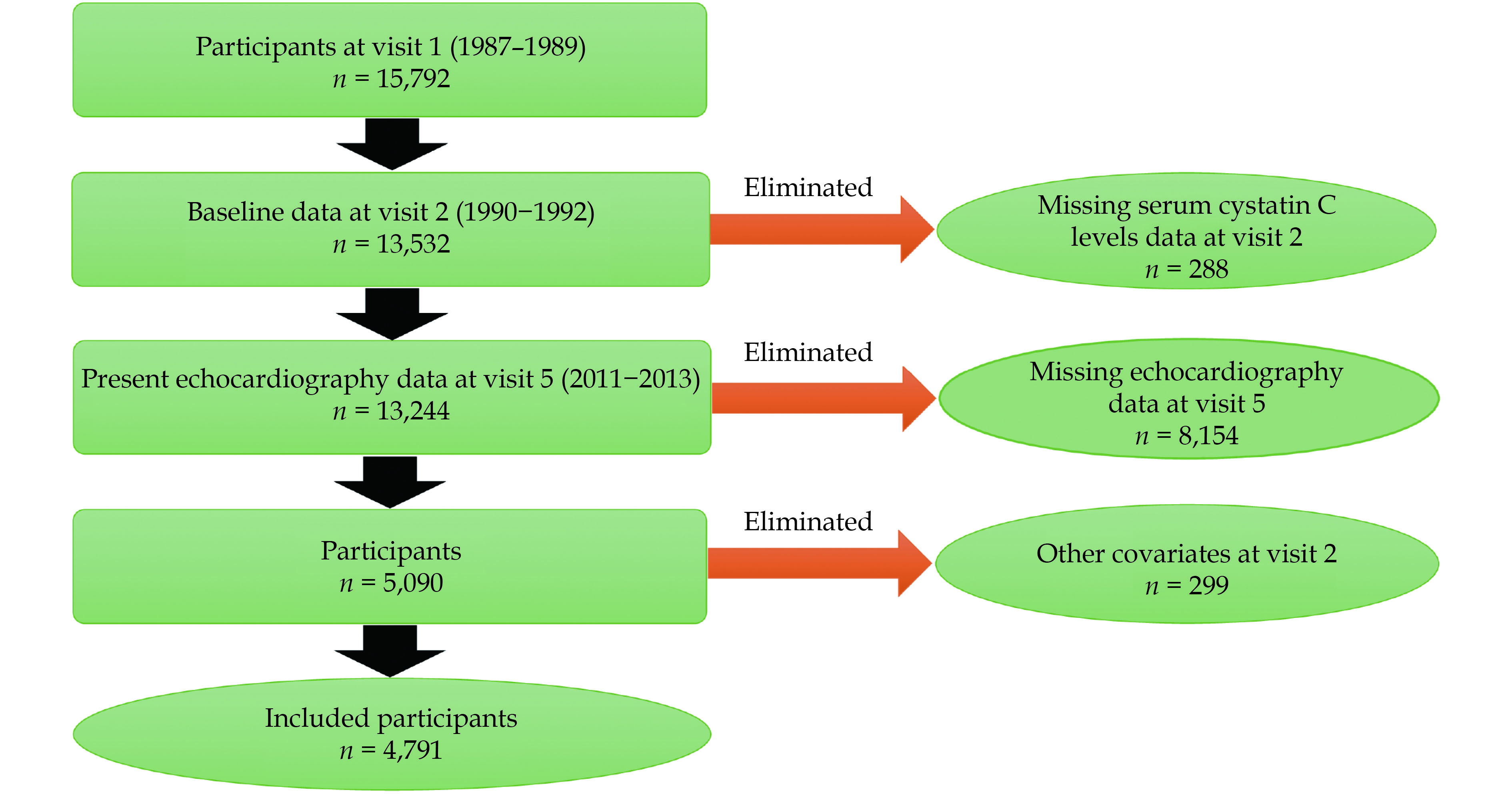
The ARIC cohort study is a population-based, prospective, cohort study of cardiovascular risk factors in the four United States communities (Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and Minneapolis suburbs, Minnesota), initially consisting of 15,792 participants, aged 45−64 years, recruited between 1987 and 1989 (visit 1). Four sub-sequent study visits were conducted: visit 2 (1990−1992), visit 3 (1993−1995), visit 4 (1996−1998), and visit 5 (2011−2013); 6,538 participants (age: 67−91 years) returned for a visit 5 that included the questionnaire survey, laboratory testing, and a comprehensive echocardiographic examination. Participants are followed up by annual or semiannual telephone interviews and active surveillance at ARIC cohort study community hospitals. Further details about the ARIC cohort study design have been previously described.[13] For this study, we included baseline participants (n = 13,532). We excluded participants with missing echocardiographic measurements of aortic valve (AV) function (n = 8,154) identified on echocardiography at visit 5, missing baseline serum CysC levels measurements (n = 288), and other covariates (n = 299). Overall, a total of 4,791 participants were included in this study (Figure 1).
The analytic data methods and study materials will not be made available to other researchers to replicate the results or replicate the procedure because of human subjects’ restrictions. ARIC cohort study data are available for distribution to outside researchers through the ARIC Study Coordinating Center at the University of North Carolina–Chapel Hill to request overall ARIC Study data access (https://sites.cscc.unc.edu/aric).
All assays were performed in serum specimens obtained from participants in 1990−1992 during visit 2. Serum creatinine was measured in samples with a modified kinetic Jaffe reaction. The reliability coefficient for 439 blinded quality-control replicates was 0.95, creatinine values were calibrated to the Cleveland Clinic Laboratory.[14] Serum CysC levels was measured from stored frozen samples collected by particle-enhanced immune nephelometric assay (N Latex CysC, Siemens Healthcare Diagnostics, Deerfield, Illinois, USA) with a BNII nephelometer, the reliability coefficient for 421 blinded quality-control replicates of CysC was 0.65, but it was 0.94 after removing 10 pair outliers. CysC was calibrated to the Cleveland Clinic Laboratory after a relatively constant difference of 16% was found between ARIC and Cleveland Clinic Laboratory values (Cleveland Clinic1.16ARIC).[15] Estimated glomerular filtration rate (eGFR) based on creatinine (eGFRcreat) was calculated from the CKD Epidemiology Collaboration equation for creatinine, and eGFR by CysC (eGFRcys) was estimated with the CKD Epidemiology Collaboration equation for cystatin: eGFRcys (mL/min per 1.73 m2) = 127.7 × CysC (mg/dL)−1.17 × age−0.13 × 0.91 (if female) × 1.06 (if black).[7,16]
A detailed method for echocardiography at visit 5 of the ARIC cohort study have been previously published.[17] All the examinations were performed by certified sonographers at the four Fifield centres, using the uniform equipment (Philips iE33 Ultrasound system) and following a standardized image acquisition protocol.[18] This protocol included pulse-wave Doppler assessment of the left ventricular outflow tract, continuous-wave Doppler assessment of flow velocities across the AV, and the AV’s assessment of the left ventricular outflow tract in the parasternal long-axis view and short-axis view. Analysts who were blinded to participant characteristics performed quantitative measures at a dedicated Echocardiography Reading Center. The same analyst performed any given measure for all echocardiographic studies. Reproducibility metrics for crucial measures of cardiac structure and function have been previously published.[17]
The AVD classification has been well established using the aortic peak velocity, the American Heart Association/American College of Cardiology guideline for the management of patients with valvular heart disease has been the most adopted.[19–21] The hemodynamic classification of AVD is as follows: (1) normal: peak aortic velocity < 1.5 m/s; (2) aortic sclerosis: peak aortic velocity ≥ 1.5 m/s to < 2.0 m/s; (3) mild stenosis: peak aortic velocity ≥ 2.0 m/s to < 3.0 m/s; (4) moderate stenosis: ≥ 3.0 m/s to < 4.0 m/s; and (5) severe stenosis ≥ 4.0 m/s. We designed moderate or severe subgroups (merged moderate stenosis and severe stenosis) for this study as peak aortic velocity ≥ 3.0 m/s for analysis due to moderate and severe stenosis cases were relatively infrequent.
CysC was measured during visit 2 (1990−1992) for the primary analysis, and AS incidence was evaluated at visit 5. Information on characteristics that might confound our outcome was obtained from the records of 1990−1992, including age, sex, and race (self-reported), smoking status, drinking status, body mass index (BMI), waist-to-hip ratio. Diabetes mellitus (DM) was defined as fasting blood glucose ≥ 126 mg/dL, non-fasting blood glucose ≥ 200 mg/dL, use of antidiabetic medicines, or self-reported physician diagnosis of DM. Hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, or blood pressure medication use in the past two weeks. Low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein(a), apolipoprotein(b) [apo(b)], total triglycerides, C-reactive protein, the presence or absence of clinical disease [prevalence of myocardial infarction, stroke was identified by six associated symptoms (speech, vision, double vision, numbness, paralysis, and dizziness)] corresponding to the specific artery disease, CHD. Echocardiogram measurements of ejection fraction, aortic peak velocity, means aortic valve gradient, systolic blood pressure, and diastolic blood pressure was also recorded.
To evaluate the associated between serum CysC levels and the outcomes, we used quintiles (Q1–Q5) cut-off point of 20th, 40th, 60th, 80th percentiles, and examined the adjustment variable distribution among CysC quintiles groups. As appropriate, baseline characteristics of participants were compared using the one-way ANOVA test, the Pearson’s chi-squared test, and the Kruskal-Wallis H test. Continuous variables are presented as mean ± SD, and categorical variables are presented as percentage. We used multivariable logistic regression models to assess the association between baseline serum CysC levels and the risk of AS. Model 1 was adjusted for age, sex, and race; model 2 was adjusted for variables in model 1 plus BMI, smoking status, and drinking status; and model 3 was adjusted for variables in model 2 plus HDL-C, LDL-C, creatinine, hypertension, DM, coronary artery disease, and apo(b). We used restricted cubic spline models with four knots to assess the dose-response association between serum CysC levels (as continuous variables) and AVD. Subgroup analysis was performed to evaluate the effect stratified by prespecified risk factors and the potential interaction effect. Two-sided P-value < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS 25.0 (SPSS Inc., IBM, Chicago, IL, USA).
Overall, a total of 4,791 consecutive ARIC participants (mean age: 54.8 ± 5.0 years, females: 57.6%, blacks: 8.2%) were included in this study, and a mean serum CysC levels was 0.80 ± 0.20 mg/L (Table 1). During a follow-up of 21 years, the mean hemodynamic peak aortic velocity was 1.3 ± 0.4 m/s, we identified 736 cases (15.4%) of aortic sclerosis, 194 cases (4.0%) of mild stenosis, and 42 cases (0.7%) of moderate-to-severe stenosis. In all patients, the serum CysC levels of Q1 was ≤ 0.91 mg/L (n = 958), Q2 was 0.94−2.26 mg/L (n = 1,065), Q3 was 2.26−4.83 mg/L (n = 936), Q4 was 4.83−9.21 mg/L (n = 880), and Q5 was ≥ 9.21 mg/L (n = 952) (Figure 2).
| Variables | Total (n = 4,791) | ≤ 0.91 mg/L (n = 958) | 0.94−2.26 mg/L (n = 1,065) | 2.26−4.83 mg/L (n = 936) | 4.83−9.21 mg/L (n = 880) | ≥ 9.21 mg/L (n = 952) | P-value |
| Age, yrs | 54.8 ± 5.0 | 53.0 ± 4.2 | 54.2 ± 4.8 | 54.8 ± 5.0 | 55.7 ± 5.0 | 56.5 ± 5.0 | < 0.001 |
| Serum cystatin C, mg/L | 0.80 ± 0.20 | 0.60 ± 0.10 | 0.80 ± 0.02 | 0.83 ± 0.02 | 0.90 ± 0.02 | 1.10 ± 0.13 | < 0.001 |
| Body mass index, kg/m2 | 27.4 ± 4.8 | 25.7 ± 4.4 | 26.8 ± 4.5 | 27.5 ± 4.4 | 28.0 ± 4.7 | 29.2 ± 5.4 | < 0.001 |
| Waist-to-hip ratio | 0.91 ± 0.08 | 0.87 ± 0.09 | 0.90 ± 0.08 | 0.91 ± 0.08 | 0.93 ± 0.07 | 0.94 ± 0.07 | < 0.001 |
| Race | < 0.001 | ||||||
| White | 3,918 (81.8%) | 725 (18.5%) | 835 (21.3%) | 793 (20.2%) | 741 (18.9%) | 824 (21.0%) | |
| Black | 8,731 (8.2%) | 233 (26.7%) | 230 (26.3%) | 143 (16.4%) | 139 (14.7%) | 128 (14.7%) | |
| Sex | < 0.001 | ||||||
| Male | 2,030 (42.4%) | 206 (10.1%) | 431 (21.2%) | 432 (21.3%) | 454 (22.4%) | 507 (25.0%) | |
| Female | 2,761 (57.6%) | 752 (27.2%) | 634 (23.0%) | 504 (18.3%) | 426 (15.4%) | 445 (16.1%) | |
| Smoking status | < 0.001 | ||||||
| Current | 730 (15.2%) | 106 (14.5%) | 138 (18.9%) | 152 (20.8%) | 144 (19.7%) | 190 (26.0%) | |
| Former | 1,889 (39.4%) | 359 (19.0%) | 438 (23.2%) | 379 (20.1%) | 349 (18.5%) | 364 (19.3%) | |
| Never | 2,172 (45.3%) | 493 (22.7%) | 489 (22.5%) | 405 (18.6%) | 387 (17.8%) | 398 (18.3%) | |
| Drinking status | 0.01 | ||||||
| Current | 3,043 (63.5%) | 626 (20.6%) | 698 (22.9%) | 606 (19.9%) | 538 (17.7%) | 575 (18.9%) | |
| Former | 747 (15.6%) | 110 (14.7%) | 155 (20.7%) | 148 (19.8%) | 153 (20.5%) | 181 (24.2%) | |
| Never | 100 (20.9%) | 222 (22.2%) | 212 (21.2%) | 182 (18.2%) | 189 (18.9%) | 196 (19.6%) | |
| Glucose, mmol/L | 5.9 ± 1.5 | 5.9 ± 2.0 | 5.9 ± 1.6 | 5.8 ± 1.3 | 5.8 ± 1.1 | 5.9 ± 1.0 | 0.191 |
| Total cholesterol, mmol/L | 5.3 ± 0.9 | 5.3 ± 0.9 | 5.3 ± 0.9 | 5.3 ± 0.9 | 5.4 ± 0.9 | 5.3 ± 1.0 | 0.268 |
| High-density lipoprotein cholesterol, mmol/L | 1.3 ± 0.4 | 1.5 ± 0.4 | 1.4 ± 0.4 | 1.3 ± 0.4 | 1.24 ± 0.39 | 1.14 ± 0.36 | < 0.001 |
| Low-density lipoprotein cholesterol, mmol/L | 3.4 ± 0.9 | 3.2 ± 0.9 | 3.4 ± 0.9 | 3.4 ± 0.9 | 3.5 ± 0.9 | 3.4 ± 0.9 | < 0.001 |
| Apolipoprotein(a), mmol/L | 1,314.7 ± 308.5 | 1,434.9 ± 313.7 | 1,344.8 ± 303.7 | 1,303.9 ± 302.8 | 1,282.9 ± 302.0 | 1,200.3 ± 270.1 | < 0.001 |
| Apolipoprotein(b), mmol/L | 925.7 ± 264.1 | 883.1 ± 253.8 | 920.8 ± 271.4 | 936.1 ± 268.8 | 941.1 ± 259.1 | 949.5 ± 261.2 | < 0.001 |
| Triglycerides, mmol/L | 1.4 ± 0.7 | 1.2 ± 0.6 | 1.3 ± 0.7 | 1.4 ± 0.7 | 1.5 ± 0.7 | 1.6 ± 0.8 | < 0.001 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 97.7 ± 13.6 | 106.4 ± 11.7 | 100.8 ± 10.8 | 98.2 ± 11.1 | 94.4 ± 12.3 | 87.8 ± 14.4 | < 0.001 |
| Uric acid, mg/dL | 5.0 ± 1.3 | 4.3 ± 1.1 | 4.8 ± 1.2 | 5.1 ± 1.2 | 5.4 ± 1.2 | 5.7 ± 1.3 | < 0.001 |
| Creatinine, mg/dL | 0.8 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.2 | < 0.001 |
| C-reactive protein, mg/dL | 4.0 ± 7.0 | 3.4 ± 5.0 | 4.0 ± 6.4 | 4.0 ± 6.1 | 4.0 ± 6.1 | 4.8 ± 9.7 | 0.001 |
| Diabetes mellitus | 231 (4.8%) | 58 (25.1%) | 47 (20.3%) | 40 (17.3%) | 41 (17.7%) | 45 (19.5%) | 0.374 |
| Hypertension | 1,189 (24.8%) | 189 (15.9%) | 232 (19.5%) | 202 (17.0%) | 237 (19.9%) | 329 (27.7%) | < 0.001 |
| Coronary heart disease | 118 (2.5%) | 12 (10.2%) | 12 (10.2%) | 22 (18.6%) | 26 (22.0%) | 46 (39.0%) | < 0.001 |
| Stroke | 34 (0.7%) | 5 (14.7%) | 5 (14.7%) | 8 (23.5%) | 3 (8.8%) | 13 (38.2%) | 0.058 |
| Systolic blood pressure, mmHg | 129.3 ± 19.3 | 128.9 ± 18.7 | 129.7 ± 18.1 | 129.7 ± 20.3 | 129.1 ± 19.2 | 128.8 ± 20.3 | 0.752 |
| Diastolic blood pressure, mmHg | 65.9 ± 11.4 | 65.9 ± 11.1 | 66.6 ± 11.0 | 66.4 ± 11.4 | 65.6 ± 11.7 | 64.8 ± 11.6 | 0.005 |
| Statin use | 91 (2.0%) | 13 (14.3%) | 18 (19.8%) | 20 (22.0%) | 19 (20.9%) | 21 (23.1%) | 0.584 |
| Ejection fraction, % | 63.3 ± 12.7 | 64.2 ± 12.6 | 63.5 ± 12.2 | 63.2 ± 12.6 | 63.6 ± 11.1 | 61.8 ± 14.8 | 0.001 |
| Peak aortic velocity, m/s | 1.3 ± 0.4 | 1.3 ± 0.3 | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.4 ± 0.4 | 1.4 ± 0.4 | < 0.001 |
| Data are presented as means ± SD or n (%). All the results in this Table are unadjusted for confounding factors. | |||||||
After adjustment for multiple potential confounders, the odds ratio (OR) was per standard deviation (0.15 mg/L) associated with an increased incidence of AVD (OR = 1.15, 95% CI: 1.05−1.26, P = 0.002). In the final model, the OR for risk of AVD comparing the Q2, Q3, Q4 and Q5 quintiles of serum CysC levels with the Q1 quintile as the reference was 1.22 (95% CI: 0.95−1.56, P = 0.118), 1.37 (95% CI: 1.06−1.78, P = 0.016), 1.33 (95% CI: 1.02−1.74, P = 0.035), and 1.26 (95% CI: 0.95−1.68, P = 0.116), respectively (Table 2).
| Quintiles | Model 1 | Model 2 | Model 3 | |||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |||
| ≤ 0.91 mg/L | Reference | Reference | Reference | |||||
| 0.94−2.26 mg/L | 1.35 (1.06−1.72) | 0.017 | 1.21 (0.95−1.55) | 0.130 | 1.22 (0.95−1.56) | 0.118 | ||
| 2.26−4.83 mg/L | 1.62 (1.27−2.07) | < 0.001 | 1.35 (1.05−1.55) | 0.020 | 1.37 (1.06−1.78) | 0.016 | ||
| 4.83−9.21 mg/L | 1.65 (1.28−2.12) | < 0.001 | 1.31 (1.01−1.69) | 0.042 | 1.33 (1.02−1.74) | 0.035 | ||
| ≥ 9.21 mg/L | 1.73 (1.35−2.21) | < 0.001 | 1.22 (0.94−1.59) | 0.134 | 1.26 (0.95−1.68) | 0.116 | ||
| Per standard deviation, 0.15 mg/L | 1.22 (1.13−1.31) | < 0.001 | 1.11 (1.03−1.19) | 0.009 | 1.15 (1.05−1.26) | 0.002 | ||
| Model 1: adjusted for age, sex, and race. Model 2: adjusted for variables in Model 1 plus body mass index, smoking status, and drinking status. Model 3: adjusted for variables in Model 2 plus high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, creatinine, hypertension, diabetes mellitus, coronary artery disease, and apolipoprotein(b). CI: confidence interval; OR: odds ratio. | ||||||||
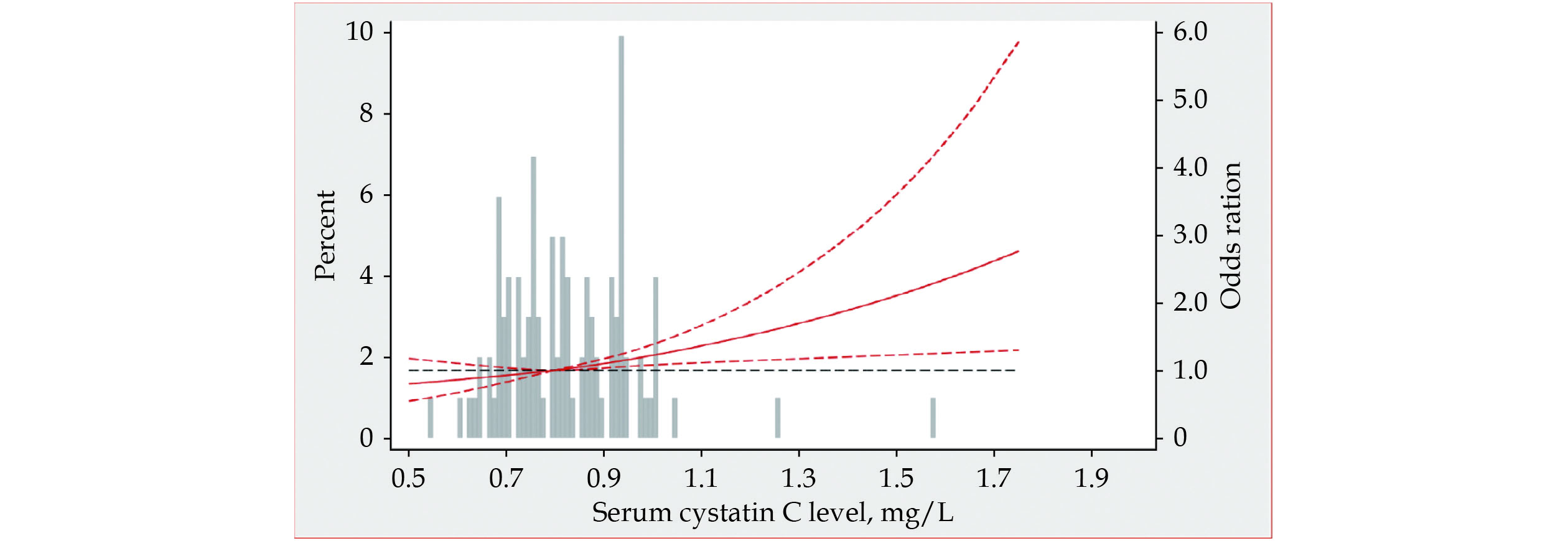
Figure 3 shows a restricted spline curve that determines the associations between adjusted OR of AVD at baseline by serum CysC levels in the ARIC. The OR was computed with the serum CysC levels of 0.8 mg/L as the reference. Using serum CysC levels measurement (as continuous variables). Consistent with quintile groups of sample distribution, the risk of hemodynamically classified AVD increases linearly with increased CysC concentration.
Figure 4 shows a forest plot that summarizes prespecified risk factors for potential interaction. All analyses were adjusted for age, sex, race, BMI, smoking status, drinking status, HDL-C, LDL-C, apo(b), creatinine, hypertension, DM, coronary artery disease, and stroke measured during baseline visit 2 (1990−1992). P-value and Pinteraction-value are shown. Variables were divided into sex (male and female), age (< 50 years, 50−60 years, and ≥ 60 years), race (white and black), smoking status (current, former, and never), drinking status (current, former, and never), BMI (< 25 kg/m2, 25−30 kg/m2, and ≥ 30 kg/m2), creatinine (< 0.73 mg/dL and ≥ 0.73 mg/dL), DM (yes and no), hypertension (yes and no), and sensitivity analyses excluding CHD and stroke. Results were similar when stratified by age, sex, race, smoking status, drinking status, BMI, creatinine, DM, hypertension, and sensitivity analyses of CHD and stroke, all Pinteraction-value > 0.05. Similarly, the associations were more significant in participants with higher creatinine (Pinteraction-value = 0.004) and the black people subgroup (Pinteraction-value = 0.032).
This large-scale population-based prospective cohort study found that an increased serum CysC level is independently associated with AS. However, participants with extremely high serum CysC levels were P-value > 0.05 for correlation for risk of AVD; hence, CysC seems to show a protective effect in this group. These associations were independent of traditional, clinical, and cardiovascular disease risk factors and were similar in male, female, whites, and blacks; in individuals with or without a history of cardiovascular disease or hypertension and kidney dysfunction. To the best of our knowledge, this is the first prospective cohort study with a long-term follow-up period to assess this association between serum CysC levels and AS.
Moderate or severe valvular heart disease affects approximately 2.5% of the United States population and increases in prevalence with respect to age in nearly 3% of people ≥ 65 years.[5,22,23] Regarding the growth in the global economy, the incidence is expected to increase in the coming years.[5,24] CysC is a significant biomarker secreted by all nucleated cells, the most abundant and potent inhibitor of cysteine proteases. It is a low molecular mass protein (13.4 kDa) and is freely filtered at the glomerulus and then reabsorbed and fully catabolized but not secreted by proximal renal tubules.[8] Recent studies have demonstrated that CysC plays an essential role in vascular remodelling, coronary artery calcification and pro-inflammatory, and is associated with adverse cardiovascular outcomes among the elderly in the population.[8,25]
The results of our study provide important additional insights to the literature regarding the correlation between AVD and varying serum CysC levels. Our results follow several extensive studies demonstrating a similar association of serum CysC levels on AVD progression, CHD and adverse stroke outcomes. Our result is signified from a study investigating electrolytic cathepsins S, K, and V, and CysC role in extracellular matrix remodelling of the stenotic aortic valves. Demonstrating that the stenotic aortic valves increased the expression and activity of electrolytic cathepsins S, K, and V, and CysC would ultimately accelerate the destruction of aortic valvular extracellular matrix, which cascades the progression of the AS.[26]
Serum CysC level is an established measurement of renal function and a stronger predictor of the risk of death and cardiovascular events in elderly persons than creatinine.[27] This concept was further strengthened by a study conducted by Onopiuk, et al.[28] in the elderly population with a significant correlation. Regarding the Q1 quintile as the reference, the OR for risk was 1.22 (95% CI: 0.95−1.56, P = 0.118), 1.37 (95% CI: 1.06−1.78, P = 0.016), and 1.33 (95% CI: 1.02−1.74, P = 0.035) with respect to the Q2, Q3, Q4 and Q5 quintiles. The OR increases linearly with increments in serum CysC levels in model 3. However, respective to the Q5 quintile, the OR was 1.26 (95% CI: 0.95−1.68, P = 0.116). The exact pathophysiology process and research explaining this outcome among elderly patients with extremely high serum CysC levels (OR = 1.26, 95% CI: 0.95–1.68, P = 0.116) and AVD displayed in our study are unknown. However, we propose multifactorial and suggest further studies to strengthen our findings. The report by Yang, et al.[29] suggest the effect of CysC on the cardiovascular outcome is independent of eGFR or creatinine. The participants enrolled in our study have an overall eGFR of 97.7 ± 13.6 mL/min per 1.73 m2 (> 60 mL/min per 1.73 m2). Similarly, suggesting that CysC has a similar effect in patients with normal kidney functions.[25] Furthermore, the elevation of serum CysC levels in CKD may increase the risk of AVD through several mechanisms. Individuals with CKD are more likely to develop hypertension and have more inadequate control of their blood pressure,[30,31] which is a significant AS risk factor. The resulting expansion of the extracellular fluid might result in left ventricular hypertrophy, poor ventricular compliance,[32] and eventually increased fibrosis, an established predictor of AS.[31]
AS in elderly populations has been related to a risk of mortality and incidence of cardiovascular events.[23] The prevention, treatment, and halt of AS disease progressions are challenging because the disease mechanism, risk factors inducing disease progression, and causative risk factors are not well established.[23] The current widely available treatment of severe stenosis in high-risk patients is aortic valve replacement.[33] Lipid-lowering medication such as statins has shown no impact in slowing aortic valve stenosis or calcification progression,[4] whereas PCSK9 has shown a promising result in lowering serum LDL-C. Nonetheless, it is still under-study for its efficacy in haltering AS disease progression.[5,34] To further understand the association between CysC and AS or sclerosis, future studies should illustrate the role of serum CysC levels in AVD progression to improve clinical decision-making in evaluating the risk-benefit and tradeoffs in prescribing medication, administration of intravenous contrast material, or surgical procedures.
This study has significant strengths. We used a large community-based bi-racial cohort with a long (≥ 20 years) follow-up duration and adequate AS and sclerosis events to test our hypotheses. With the extensive and definite estimation of covariates, the design of ARIC cohort study allowed us to perform the comprehensive statistical adjustment and reduce confounding as much as possible. There are several limitations of this study that should be acknowledged. Firstly, the relatively few participants (0.7%) with moderate-to-severe AS limit the precision of these predominance estimates. Secondly, our data show baseline participants approximately aged 48−70 years (visit 2), so we do not know whether serum CysC levels would be a possible AS predictor among younger persons. Last but not least, the participants alive at the start of visit 5, 38% of them rejected participating, presumably resulting in selection bias.
In conclusion, the present study demonstrates that serum CysC level is independently associated with an increased risk of hemodynamic aortic sclerosis and stenosis. However, this association does not extend to patients with extremely high serum CysC levels. Factors behind this phenomenon are likely multifactorial and necessitate further investigations. We did not outline a set of criteria for including patients with extremely high serum CysC levels based on the potential factors. Further studies are encouraged to assess these issues and further evaluate the pathophysiological role of serum CysC levels in aortic valve stenosis.
This study was supported by the National Natural Science Foundation of China (No.81600206), and the Natural Science Foundation of Guangdong Province (2016A030310140). All authors had no conflicts of interest to disclose. The authors gratefully thank the staff and participants of the Atherosclerosis Risk in Communities Study for their significant contributions.
| [1] |
Akahori H, Tsujino T, Masuyama T, et al. Mechanisms of aortic stenosis. J Cardiol 2018; 71: 215−220. doi: 10.1016/j.jjcc.2017.11.007
|
| [2] |
Enas EA, Varkey B, Dharmarajan TS, et al. Lipoprotein(a): an independent, genetic, and causal factor for cardiovascular disease and acute myocardial infarction. Indian Heart J 2019; 71: 99−112. doi: 10.1016/j.ihj.2019.03.004
|
| [3] |
Grimard BH, Safford RE, Burns EL. Aortic stenosis: diagnosis and treatment. Am Fam Physician 2016; 93: 371−378.
|
| [4] |
Lindman BR, Dweck MR, Lancellotti P, et al. Management of asymptomatic severe aortic stenosis: evolving concepts in timing of valve replacement. JACC Cardiovasc Imaging 2020; 13: 481−493. doi: 10.1016/j.jcmg.2019.01.036
|
| [5] |
Capoulade R, Cariou B. Editorial commentary: Lp(a) and calcific aortic valve stenosis: direct LPA targeting or PCSK9-lowering therapy? Trends Cardiovasc Med 2021; 31: 312−314. doi: 10.1016/j.tcm.2020.06.009
|
| [6] |
Sengeløv M, Cheng S, Biering-Sørensen T, et al. Ideal cardiovascular health and the prevalence and severity of aortic stenosis in elderly patients. J Am Heart Assoc 2018; 7: e007234. doi: 10.1161/JAHA.117.007234
|
| [7] |
Vollema EM, Prihadi EA, Ng ACT, et al. Prognostic implications of renal dysfunction in patients with aortic stenosis. Am J Cardiol 2020; 125: 1108−1114. doi: 10.1016/j.amjcard.2019.12.040
|
| [8] |
Salgado JV, Souza FL, Salgado BJ. How to understand the association between cystatin C levels and cardiovascular disease: imbalance, counterbalance, or consequence? J Cardiol 2013; 62: 331−335. doi: 10.1016/j.jjcc.2013.05.015
|
| [9] |
Verbree-Willemsen L, Zhang YN, Ibrahim I, et al. Extracellular vesicle cystatin C and CD14 are associated with both renal dysfunction and heart failure. ESC Heart Fail 2020; 7: 2240−2249. doi: 10.1002/ehf2.12699
|
| [10] |
Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005; 352: 2049−2060. doi: 10.1056/NEJMoa043161
|
| [11] |
van der Laan SW, Fall T, Soumaré A, et al. Cystatin C and cardiovascular disease: a mendelian randomization study. J Am Coll Cardiol 2016; 68: 934−945. doi: 10.1016/j.jacc.2016.05.092
|
| [12] |
Wang S, Liu Q, Guo F, et al. Clinical utility of serum cystatin C for prediction of multi-vessel disease by coronary angiography in type 2 diabetes mellitus patients withnormal renal function. BMC Cardiovasc Disord 2020; 20: 183. doi: 10.1186/s12872-020-01475-4
|
| [13] |
The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989; 129: 687−702. doi: 10.1093/oxfordjournals.aje.a115184
|
| [14] |
Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis 2002; 39: 920−929. doi: 10.1053/ajkd.2002.32765
|
| [15] |
Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol 2003; 41: 47−55. doi: 10.1016/s0735-1097(02)02663-3
|
| [16] |
Alonso A, Lopez FL, Matsushita K, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011; 123: 2946−2953. doi: 10.1161/CIRCULATIONAHA.111.020982
|
| [17] |
Shah AM, Cheng S, Skali H, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging 2014; 7: 173−181. doi: 10.1161/CIRCIMAGING.113.000736
|
| [18] |
Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr 2009; 10: 1−25. doi: 10.1093/ejechocard/jen303
|
| [19] |
Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: 2440−2492. doi: 10.1161/CIR.0000000000000029
|
| [20] |
Côté N, Simard L, Zenses AS, et al. Impact of vascular hemodynamics on aortic stenosis evaluation: new insights into the pathophysiology of normal flow-small aortic valve area-low gradient pattern. J Am Heart Assoc 2017; 6: e006276. doi: 10.1161/JAHA.117.006276
|
| [21] |
Writing Committee Members, Otto CM, Nishimura RA, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021; 77: 450−500. doi: 10.1016/j.jacc.2020.11.035
|
| [22] |
Reddy YNV, Nishimura RA. Evaluating the severity of aortic stenosis: a re-look at our current ‘gold standard’ measurements. Eur Heart J 2018; 39: 2656−2658. doi: 10.1093/eurheartj/ehy224
|
| [23] |
Otto CM. Aortic stenosis: even mild disease is significant. Eur Heart J 2004; 25: 185−187. doi: 10.1016/j.ehj.2003.12.010
|
| [24] |
Andell P, Li X, Martinsson A, et al. Neighborhood socioeconomic status and aortic stenosis: a Swedish study based on nationwide registries and an echocardiographic screening cohort. Int J Cardiol 2020; 318: 153−159. doi: 10.1016/j.ijcard.2020.06.034
|
| [25] |
Sugiyama H, Miyoshi T, Osawa K, et al. Serum cystatin C levels are associated with coronary artery calcification in women without chronic kidney disease. J Cardiol 2017; 70: 559−564. doi: 10.1016/j.jjcc.2017.05.001
|
| [26] |
Helske S, Syväranta S, Lindstedt KA, et al. Increased expression of elastolytic cathepsins S, K, and V and their inhibitor cystatin C in stenotic aortic valves. Arterioscler Thromb Vasc Biol 2006; 26: 1791−1798. doi: 10.1161/01.ATV.0000228824.01604.63
|
| [27] |
Beilby J, Divitini ML, Knuiman MW, et al. Comparison of cystatin C and creatinine as predictors of cardiovascular events in a community-based elderly population. Clin Chem 2010; 56: 799−804. doi: 10.1373/clinchem.2009.135962
|
| [28] |
Onopiuk A, Tokarzewicz A, Gorodkiewicz E. Cystatin C: a kidney function biomarker. Adv Clin Chem 2015; 68: 57−69. doi: 10.1016/bs.acc.2014.11.007
|
| [29] |
Yang S, Song L, Zhao L, et al. Predictive value of cystatin C in people with suspected or established coronary artery disease: a meta-analysis. Atherosclerosis 2017; 263: 60−67. doi: 10.1016/j.atherosclerosis.2017.05.025
|
| [30] |
Ravera M, Noberasco G, Re M, et al. Chronic kidney disease and cardiovascular risk in hypertensive type 2 diabetics: a primary care perspective. Nephrol Dial Transplant 2009; 24: 1528−1533. doi: 10.1093/ndt/gfn692
|
| [31] |
Ureña-Torres P, D’Marco L, Raggi P, et al. Valvular heart disease and calcification in CKD: more common than appreciated. Nephrol Dial Transplant 2020; 35: 2046−2053. doi: 10.1093/ndt/gfz133
|
| [32] |
Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003; 42: 1050−1065. doi: 10.1161/01.HYP.0000102971.85504.7c
|
| [33] |
Braverman AC. Aortic replacement for bicuspid aortic valve aortopathy: when and why? J Thorac Cardiovasc Surg 2019; 157: 520−525. doi: 10.1016/j.jtcvs.2018.06.023
|
| [34] |
Ternacle J, Côté N, Krapf L, et al. Chronic kidney disease and the pathophysiology of valvular heart disease. Can J Cardiol 2019; 35: 1195−1207. doi: 10.1016/j.cjca.2019.05.028
|
| Variables | Total (n = 4,791) | ≤ 0.91 mg/L (n = 958) | 0.94−2.26 mg/L (n = 1,065) | 2.26−4.83 mg/L (n = 936) | 4.83−9.21 mg/L (n = 880) | ≥ 9.21 mg/L (n = 952) | P-value |
| Age, yrs | 54.8 ± 5.0 | 53.0 ± 4.2 | 54.2 ± 4.8 | 54.8 ± 5.0 | 55.7 ± 5.0 | 56.5 ± 5.0 | < 0.001 |
| Serum cystatin C, mg/L | 0.80 ± 0.20 | 0.60 ± 0.10 | 0.80 ± 0.02 | 0.83 ± 0.02 | 0.90 ± 0.02 | 1.10 ± 0.13 | < 0.001 |
| Body mass index, kg/m2 | 27.4 ± 4.8 | 25.7 ± 4.4 | 26.8 ± 4.5 | 27.5 ± 4.4 | 28.0 ± 4.7 | 29.2 ± 5.4 | < 0.001 |
| Waist-to-hip ratio | 0.91 ± 0.08 | 0.87 ± 0.09 | 0.90 ± 0.08 | 0.91 ± 0.08 | 0.93 ± 0.07 | 0.94 ± 0.07 | < 0.001 |
| Race | < 0.001 | ||||||
| White | 3,918 (81.8%) | 725 (18.5%) | 835 (21.3%) | 793 (20.2%) | 741 (18.9%) | 824 (21.0%) | |
| Black | 8,731 (8.2%) | 233 (26.7%) | 230 (26.3%) | 143 (16.4%) | 139 (14.7%) | 128 (14.7%) | |
| Sex | < 0.001 | ||||||
| Male | 2,030 (42.4%) | 206 (10.1%) | 431 (21.2%) | 432 (21.3%) | 454 (22.4%) | 507 (25.0%) | |
| Female | 2,761 (57.6%) | 752 (27.2%) | 634 (23.0%) | 504 (18.3%) | 426 (15.4%) | 445 (16.1%) | |
| Smoking status | < 0.001 | ||||||
| Current | 730 (15.2%) | 106 (14.5%) | 138 (18.9%) | 152 (20.8%) | 144 (19.7%) | 190 (26.0%) | |
| Former | 1,889 (39.4%) | 359 (19.0%) | 438 (23.2%) | 379 (20.1%) | 349 (18.5%) | 364 (19.3%) | |
| Never | 2,172 (45.3%) | 493 (22.7%) | 489 (22.5%) | 405 (18.6%) | 387 (17.8%) | 398 (18.3%) | |
| Drinking status | 0.01 | ||||||
| Current | 3,043 (63.5%) | 626 (20.6%) | 698 (22.9%) | 606 (19.9%) | 538 (17.7%) | 575 (18.9%) | |
| Former | 747 (15.6%) | 110 (14.7%) | 155 (20.7%) | 148 (19.8%) | 153 (20.5%) | 181 (24.2%) | |
| Never | 100 (20.9%) | 222 (22.2%) | 212 (21.2%) | 182 (18.2%) | 189 (18.9%) | 196 (19.6%) | |
| Glucose, mmol/L | 5.9 ± 1.5 | 5.9 ± 2.0 | 5.9 ± 1.6 | 5.8 ± 1.3 | 5.8 ± 1.1 | 5.9 ± 1.0 | 0.191 |
| Total cholesterol, mmol/L | 5.3 ± 0.9 | 5.3 ± 0.9 | 5.3 ± 0.9 | 5.3 ± 0.9 | 5.4 ± 0.9 | 5.3 ± 1.0 | 0.268 |
| High-density lipoprotein cholesterol, mmol/L | 1.3 ± 0.4 | 1.5 ± 0.4 | 1.4 ± 0.4 | 1.3 ± 0.4 | 1.24 ± 0.39 | 1.14 ± 0.36 | < 0.001 |
| Low-density lipoprotein cholesterol, mmol/L | 3.4 ± 0.9 | 3.2 ± 0.9 | 3.4 ± 0.9 | 3.4 ± 0.9 | 3.5 ± 0.9 | 3.4 ± 0.9 | < 0.001 |
| Apolipoprotein(a), mmol/L | 1,314.7 ± 308.5 | 1,434.9 ± 313.7 | 1,344.8 ± 303.7 | 1,303.9 ± 302.8 | 1,282.9 ± 302.0 | 1,200.3 ± 270.1 | < 0.001 |
| Apolipoprotein(b), mmol/L | 925.7 ± 264.1 | 883.1 ± 253.8 | 920.8 ± 271.4 | 936.1 ± 268.8 | 941.1 ± 259.1 | 949.5 ± 261.2 | < 0.001 |
| Triglycerides, mmol/L | 1.4 ± 0.7 | 1.2 ± 0.6 | 1.3 ± 0.7 | 1.4 ± 0.7 | 1.5 ± 0.7 | 1.6 ± 0.8 | < 0.001 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 97.7 ± 13.6 | 106.4 ± 11.7 | 100.8 ± 10.8 | 98.2 ± 11.1 | 94.4 ± 12.3 | 87.8 ± 14.4 | < 0.001 |
| Uric acid, mg/dL | 5.0 ± 1.3 | 4.3 ± 1.1 | 4.8 ± 1.2 | 5.1 ± 1.2 | 5.4 ± 1.2 | 5.7 ± 1.3 | < 0.001 |
| Creatinine, mg/dL | 0.8 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.2 | < 0.001 |
| C-reactive protein, mg/dL | 4.0 ± 7.0 | 3.4 ± 5.0 | 4.0 ± 6.4 | 4.0 ± 6.1 | 4.0 ± 6.1 | 4.8 ± 9.7 | 0.001 |
| Diabetes mellitus | 231 (4.8%) | 58 (25.1%) | 47 (20.3%) | 40 (17.3%) | 41 (17.7%) | 45 (19.5%) | 0.374 |
| Hypertension | 1,189 (24.8%) | 189 (15.9%) | 232 (19.5%) | 202 (17.0%) | 237 (19.9%) | 329 (27.7%) | < 0.001 |
| Coronary heart disease | 118 (2.5%) | 12 (10.2%) | 12 (10.2%) | 22 (18.6%) | 26 (22.0%) | 46 (39.0%) | < 0.001 |
| Stroke | 34 (0.7%) | 5 (14.7%) | 5 (14.7%) | 8 (23.5%) | 3 (8.8%) | 13 (38.2%) | 0.058 |
| Systolic blood pressure, mmHg | 129.3 ± 19.3 | 128.9 ± 18.7 | 129.7 ± 18.1 | 129.7 ± 20.3 | 129.1 ± 19.2 | 128.8 ± 20.3 | 0.752 |
| Diastolic blood pressure, mmHg | 65.9 ± 11.4 | 65.9 ± 11.1 | 66.6 ± 11.0 | 66.4 ± 11.4 | 65.6 ± 11.7 | 64.8 ± 11.6 | 0.005 |
| Statin use | 91 (2.0%) | 13 (14.3%) | 18 (19.8%) | 20 (22.0%) | 19 (20.9%) | 21 (23.1%) | 0.584 |
| Ejection fraction, % | 63.3 ± 12.7 | 64.2 ± 12.6 | 63.5 ± 12.2 | 63.2 ± 12.6 | 63.6 ± 11.1 | 61.8 ± 14.8 | 0.001 |
| Peak aortic velocity, m/s | 1.3 ± 0.4 | 1.3 ± 0.3 | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.4 ± 0.4 | 1.4 ± 0.4 | < 0.001 |
| Data are presented as means ± SD or n (%). All the results in this Table are unadjusted for confounding factors. | |||||||
| Quintiles | Model 1 | Model 2 | Model 3 | |||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |||
| ≤ 0.91 mg/L | Reference | Reference | Reference | |||||
| 0.94−2.26 mg/L | 1.35 (1.06−1.72) | 0.017 | 1.21 (0.95−1.55) | 0.130 | 1.22 (0.95−1.56) | 0.118 | ||
| 2.26−4.83 mg/L | 1.62 (1.27−2.07) | < 0.001 | 1.35 (1.05−1.55) | 0.020 | 1.37 (1.06−1.78) | 0.016 | ||
| 4.83−9.21 mg/L | 1.65 (1.28−2.12) | < 0.001 | 1.31 (1.01−1.69) | 0.042 | 1.33 (1.02−1.74) | 0.035 | ||
| ≥ 9.21 mg/L | 1.73 (1.35−2.21) | < 0.001 | 1.22 (0.94−1.59) | 0.134 | 1.26 (0.95−1.68) | 0.116 | ||
| Per standard deviation, 0.15 mg/L | 1.22 (1.13−1.31) | < 0.001 | 1.11 (1.03−1.19) | 0.009 | 1.15 (1.05−1.26) | 0.002 | ||
| Model 1: adjusted for age, sex, and race. Model 2: adjusted for variables in Model 1 plus body mass index, smoking status, and drinking status. Model 3: adjusted for variables in Model 2 plus high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, creatinine, hypertension, diabetes mellitus, coronary artery disease, and apolipoprotein(b). CI: confidence interval; OR: odds ratio. | ||||||||