| Citation: | Cheng-Duo ZHANG, Xin-Ye XU, Li-Jun GUO, Wei GAO. A case report of significant progression after FFR-guided deferred PCI[J]. Journal of Geriatric Cardiology, 2020, 17(10): 649-652. DOI: 10.11909/j.issn.1671-5411.2020.10.008 |
In recent years, the use of coronary functional evaluation derived by fractional flow reserve (FFR) to guide percutaneous coronary intervention (PCI) treatment has been recommended by several mainstream guidelines. Typically, FFR > 0.80 in coronary artery indicates the lesions do not affect the coronary blood flow. Therefore, instead of PCI treatment, intensive drug therapy might be more beneficial. However, some lesions with an FFR > 0.80 still progress during the course of follow-up, with increased incidence of cardiovascular events. Additionally, left anterior descending coronary artery (LAD) and non-LAD lesions with an FFR > 0.80 show different characteristics of progress. Here, we present a highly representative case of coronary artery disease with PCI treatment guided by FFR, hoping that will enable a more in-depth understanding of FFR.
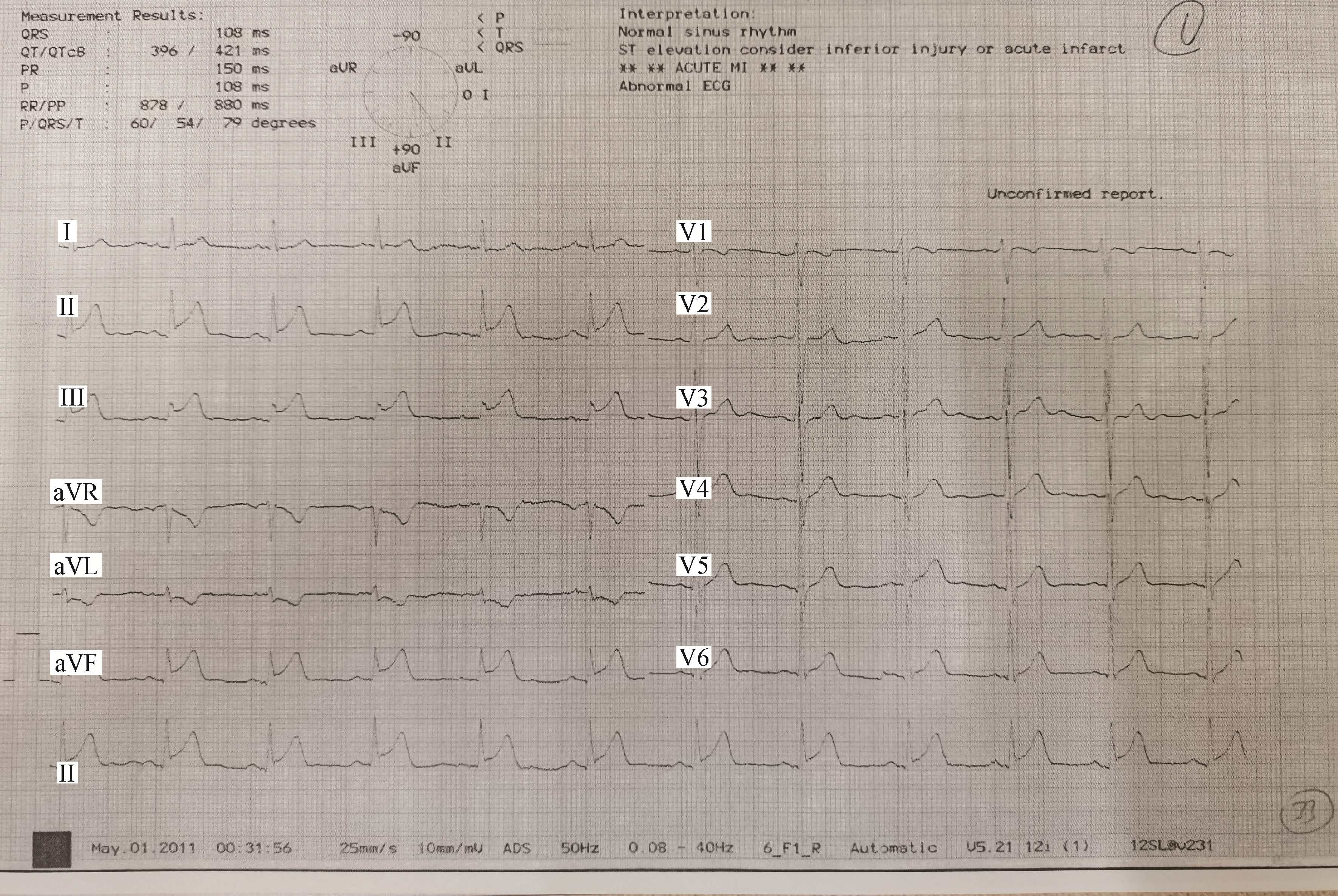
We describe a 55-year-old male patient who first experienced persistent angina pectoris in a resting state nine years ago in 2011. He smoked 20 cigarettes a day for 30 years, suffered from hyperlipidemia and regularly used rosuvastatin 10 mg once a day for one year. His father was diagnosed with coronary heart disease at the age of 40 years. The electrocardiogram showed ST-segment elevation and ST-segment depression in corresponding leads (Figure 1). He had elevated troponin T and creatine kinase-myocardial band, and was diagnosed with acute inferior myocardial infarction. Acute angiography showed total occlusion of the distal right coronary artery (RCA) which was considered the culprit lesion. An intermediate lesion in the mid-RCA, and diffused long intermediate lesions in the LAD and left circumflex coronary artery (LCX) were also observed. A drug-eluting stent was successfully placed in the distal-RCA during primary PCI, while FFR and intravascular ultrasound (IVUS) evaluations for LAD and LCX were performed five days later.
The LCX evaluation showed a distal intracoronary pressure (Pd)/aortic pressure (Pa) of 1.0 at rest, and an FFR of 0.81 was measured after the intravenous infusion of adenosine triphosphate at a rate of 160 µg per kg/min. IVUS results revealed the minimum lumen area (MLA) of 2.9 mm2 and the plaque burden (PB) of 74% in LCX lesions (Figure 2A). When it referred to LAD, the resting Pd/Pa was 0.87 and FFR was 0.76, with MLA of 2.2 mm2 and PB of 66% consequently. According to the FFR result, the revascularization was deferred in LCX and a drug-eluting stent (3.0 mm × 36 mm; Partner, Lepu Medical Technology, Beijing, China) was inserted into the middle section of the LAD. The post-stenting Pd/Pa and FFR of the LAD were 0.90 and 0.84 separately (Figure 3). The patient quit smoking on discharge and received regular dual antiplatelet therapy with aspirin and clopidogrel. Clopidogrel therapy was withdrawn after 12 months. The treatment with fosinopril 5 mg and rosuvastatin 10 mg per day was also started. Low-density lipoprotein cholesterol (LDL-C) levels of 2.06 mmol/L, glycated hemoglobin of 5.4% were recorded at discharge.
In 2014, the patient developed exertional angina pectoris and admitted to Cardiology Department of Peking University Third Hospital. Re-examination of coronary angiography revealed a significant exacerbation of mid-RCA lesion which had progressed to approximate 90% in diameter stenosis. A 50%-60% in-stent stenosis of the proximal LAD stent, and a 70% stenosis of mid-LCX were also observed. PCI was performed at RCA middle segment lesions. Functional assessment revealed the FFR of LAD was 0.75 and the FFR of LCX was 0.64. Accordingly, revascularization was deferred in LAD and performed in LCX with drug-eluting stent (2.75 mm × 38 mm; XIENCE PRIME, Abbott, USA) (Figure 4). The post-stenting FFR recovered to 0.94. The patient continued standardized medicine treatment with the LDL-C level of 2.07 mmol/L. During another five-year follow-up, there was no more relapse or rehospitalization, whereas the result of treadmill exercise test was negative in 2019.
FFR is determined as the ratio of Pd to Pa at maximum hyperemia which could identify whether the lesion induces myocardial ischemia in the coronary artery. If there is no non-invasive evidence of ischemia, FFR is recommended to measure the blood flow reserve in intermediate coronary stenosis or a lumen diameter of stenosis < 90%.[1, 2] Both FAME and FAME 2 studies used 0.80 as the cut-off value for judging myocardial ischemia. However, in the FAME 2 study, there were 332 patients with FFR > 0.80 who received drug treatment, while the overall cardiovascular event rate reached 15.7% in five years.[3] Although lesions with FFR > 0.80 are not a cause of myocardial ischemia in terms of blood flow function, they are still associated with a considerable incidence of cardiovascular events. The delayed lesion intervention (DLI) incidence within the first year of deferring revascularization based on the FFR ranged from 2.5%-11% in previous studies. During a follow-up period of 4.0 ± 2.3 years, the cumulative incidence of DLI after delayed therapy was 18%.[4]
As for the patient, there are some factors for DLI in the LCX lesions even though FFR > 0.80. The application of intravascular imaging would be helpful to determine further treatment strategies. Naghavi, et al.[5] put forward that superficial calcified nodules, active inflammation, thin fibrous cap and large lipid nucleus were the characteristics of vulnerable plaque through autopsy in 2003. With the application of optical coherence tomography, people thought superficial calcification was related to plaque erosion at the culprit site in acute coronary syndromes (ACS) patients.[6] Prospect and Atheroremo studies use IVUS to predict major adverse cardiovascular events (MACE) in 1-3 years. They considered MLA < 4 mm2, PB > 70% and thin-cap fibroatheroma, which is characterized by increased PB, positive remodeling of vessels, a large lipid core covered by a thin fibrous cap, are significantly related to it.[7] Since the IVUS measured a MLA of 2.9 mm2 and PB of 74% (which is over 70%) in LCX lesions, virtual histology IVUS showed a large lipid core plaque and superficial calcified nodules (Figure 2B) in 2011, those results confirmed a heavy PB with evidence of unstable plaques that could induce pathology in 2014.
Otherwise, LAD lesions treated with PCI and untreated LCX lesions in 2011 show different characteristics of progression, which suggests FFR > 0.80 is unable to reflect the composition and stability of atherosclerotic plaque in different arteries. As FFR is linked to the myocardial blood supply area, [8] when non-LAD lesions and LAD lesions show the same FFR value, the PB of non-LAD lesions may be more serious. Therefore, it is reasonable that the prognosis of non-LAD lesions is worse than that of LAD lesions in deferring revascularization guided by FFR, especially for the non-LAD lesions with FFR greater than 0.80, but in a relatively low region.[9] This situation suggests that we need a specific gray area for non-LAD lesions which may indicate further intracoronary imaging evaluation.
In addition, microvascular dysfunction in ACS may affect maximum hyperemia for six months, resulting in overestimation of the FFR. Based on deferring revascularization guided by the FFR, the risk of delayed lesion failure is significantly increased for every 0.01 decrease in the FFR in ACS patients.[10] An observational analysis comparing ACS and stable coronary artery disease patients suggested the optimal critical value for predicting future MACE is 0.81 in stable coronary artery disease patients but 0.84 in ACS patients.[11] Hemodynamic evaluation of the patient revealed the FFR of the LCX is 0.81, which is a critical value that may be overestimated. Thus, the deferring revascularization of the LCX increases the risk of DLI.
In summary, FFR > 0.80 is not absolutely safe. It is meaningful to combine hemodynamic calculation and intravascular imaging to guide the treatment strategy for intermediate coronary stenosis when the FFR reaches a critical value in addition to secondary prevention treatment. However, more clinical studies are still needed to verify the importance of these scientific means in risk stratification and subsequent treatment guidance in the future.
All authors had no conflicts of interest to disclose.
| [1] |
Nam CW, Yoon HJ, Cho YK, et al. Outcomes of percutaneous coronary intervention in intermediate coronary artery disease: fractional flow reserve-guided versus intravascular ultrasound-guided. JACC Cardiovasc Interv 2010; 3: 812-817. doi: 10.1016/j.jcin.2010.04.016
|
| [2] |
Ihdayhid AR, Yong A, Harper R, et al. A practical guide for fractional flow reserve guided revascularisation. Heart Lung Circ 2018; 27: 406-419. doi: 10.1016/j.hlc.2017.09.017
|
| [3] |
Lotfi A, Jeremias A, Fearon WF, et al. Expert consensus statement on the use of fractional flow reserve, intravascular ultrasound, and optical coherence tomography: a consensus statement of the Society of Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2014; 83: 509-518. doi: 10.1002/ccd.25222
|
| [4] |
Depta JP, Patel JS, Novak E, et al. Risk model for estimating the 1-year risk of deferred lesion intervention following deferred revascularization after fractional flow reserve assessment. Eur Heart J 2015; 36: 509-515. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=94fd2e663b225547229d85891fed398e
|
| [5] |
Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part Ⅱ. Circulation 2003; 108: 1772-1778. doi: 10.1161/01.CIR.0000087481.55887.C9
|
| [6] |
Sugiyama T, Yamamoto E, Fracassi F, et al. Calcified plaques in patients with acute coronary syndromes. JACC Cardiovasc Interv 2019; 12: 531-540. doi: 10.1016/j.jcin.2018.12.013
|
| [7] |
Johnson TW, Räber L, di Mario C, et al. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J 2019; 40: 2566-2584. doi: 10.1093/eurheartj/ehz332
|
| [8] |
Leone AM, De Caterina AR, Basile E, et al. Influence of the amount of myocardium subtended by a stenosis on fractional flow reserve. Circ Cardiovasc Interv 2013; 6: 29-36. doi: 10.1161/CIRCINTERVENTIONS.112.971101
|
| [9] |
Shah K, Hou L, Ghosh B, et al. Prognostic significance of non-ischemic FFR (> 0.80) in LAD versus non-LAD lesions. J Am Coll Cardiol 2020; 75: 1453.
|
| [10] |
Masrani Mehta S, Depta JP, Novak E, et al. Association of lower fractional flow reserve values with higher risk of adverse cardiac events for lesions deferred revascularization among patients with acute coronary syndrome. J Am Heart Assoc 2015; 4: e002172. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1161/JAHA.115.002172
|
| [11] |
Achenbach S, Rudolph T, Rieber J, et al. Performing and interpreting fractional flow reserve measurements in clinical practice: an expert consensus document. Interv Cardiol 2017; 12: 97-109. doi: 10.15420/icr.2017:13:2
|