| Citation: | Please cite this article as: FAN HL, ZENG LH, CHEN PY, LIU YH, DUAN CY, HE WF, TAN N, CHEN JY, HE PC. Association of baseline hemoglobin A1c levels with bleeding in patients with non-ST-segment elevation acute coronary syndrome underwent percutaneous coronary intervention: insights of a multicenter cohort study from China. J Geriatr Cardiol 2022; 19(7): 487−497. DOI: 10.11909/j.issn.1671-5411.2022.07.004. |
Diabetes mellitus (DM) is associated with a higher risk of ischemia events, including mortality, heart failure, and stroke, in patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS).[1-3] Noticeably, patients with NSTE-ACS and DM had the worst long-term prognosis among all ACS patients.[4] Additionally, DM has been determined as the independent predictor of in-hospital bleeding events in patients with non-ST-segment elevation myocardial infarction (NSTEMI).[5,6] Although dual antiplatelet therapy (DAPT) has been identified as the core component of management of NSTE-ACS, no specific recommendation on antithrombotic pharmacotherapy for NSTE-ACS patients with DM has been made.[7,8] Thus, the optimal prediction for the prognosis of patients with NSTE-ACS is important.
Since the history of DM has been identified as the independent predictive factor for bleeding events in patients with NSTEMI,[5] HbA1c levels may be a predictive factor of bleeding in ACS patients undergoing percutaneous coronary intervention (PCI). A recent study has demonstrated that the association between baseline HbA1c levels and all-cause death or myocardial infarction in patients with ACS displays U-shape curve.[9]
The previous study has reported that higher HbA1c levels were associated with a higher incidence of bleeding events in ACS patients, including those who received invasive treatment or only medical management.[10] NSTE-ACS patients undergoing PCI who were administrated with additional anticoagulants during procedure had a higher risk of bleeding events.[11,12] Thus, the association between baseline HbA1c levels and bleeding events in NSTE-ACS patients undergoing PCI should be further investigated.
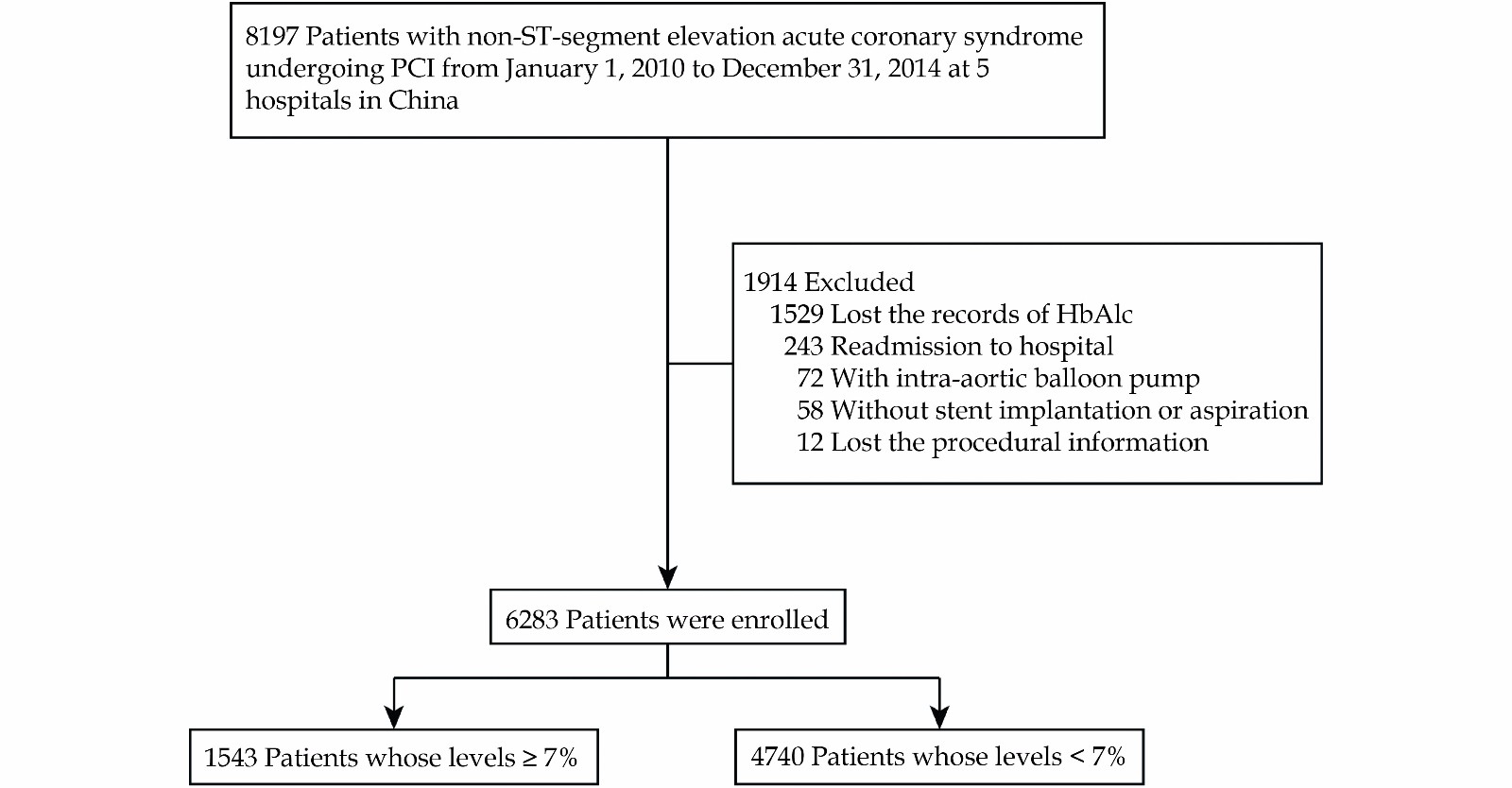
Based on our previously published multicenter cohort study,[13] this observational cohort study enrolled 8197 consecutive NSTE-ACS patients undergoing PCI from January 1, 2010 to December 31, 2014 at five centers in China. The study protocol was approved by the central ethics committee of the Guangdong Provincial People’s Hospital, Guangzhou, China, with a waiver of informed consent (No. GDREC2016210H(R1)). Central ethical approval was applicable at the other collaborating hospitals as well. The patients above 18 years and had information about the history of T2DM and admission HbA1c were enrolled. Patients were excluded if they required an intra-aortic balloon pump, did not receive stent implantation or aspiration, were readmitted to the hospital, and their information on PCI procedure was missed. Finally, 6283 patients were included in this study. Based on baseline HbA1c levels, the patients were divided into the HbA1c < 7% group and the HbA1c ≥ 7% group (Figure 1).
Data including patient demographics, laboratory test results, PCI procedural details, antithrombotic therapies, other medical treatments, and clinical events were extracted from the hospital electrical records by investigators. Follow-up was conducted by trained nurses via telephone interviews or clinical visits.
The primary outcomes were major bleeding and all-cause death during follow-up. The secondary outcomes included several in-hospital events, including all-cause death, major bleeding, any bleeding, and major adverse cardiovascular events (MACEs). The secondary outcomes also included any bleeding during follow-up.
Major bleeding and any bleeding were defined by the BARC grades 3-5 and grades 1-5,[14] respectively. MACEs were defined as the composite events of all-cause death, myocardial infarction, target vessel revascularization, and stroke.
Continuous variables following normal distribution were presented as mean ± SD, and the differences between groups were analyzed using two-sample t-tests. Non-normally distributed continuous variables were expressed as medians and interquartile ranges and were compared using Wilcoxon rank-sum tests. Categorical variables were described as a number (n) with percentage (%), and differences were analyzed by Pearson χ2 or Fisher exact test.
Logistic regression was performed as a multivariable analysis for in-hospital outcomes. Cox proportional hazards regression model was performed as a multivariable analysis for clinical outcomes during follow-up. An additive hazards model was used to detect time-varying associations. Potential confounders that were significant in the univariate analysis or clinically important. Age, sex, heart rate, systolic blood pressure, hemoglobin levels, history of DM, history of myocardial infarction, history of PCI, history of hypertension, current smoking, estimated glomerular filtration rate (eGFR), DAPT, glycoprotein Ⅱb/Ⅲa inhibitor, warfarin, parenteral anticoagulation therapy, the diagnosis of NSTE-ACS subtypes were adjusted in the multivariable models for bleeding events in hospital and during follow-up.
For adjustment of logistic regression analyses for in-hospital death and in-hospital MACEs, age, sex, history of myocardial infarction, history of stroke, chronic heart failure, eGFR, diagnosis of NSTE-ACS subtypes, coronary anatomy, the interval from admission to PCI procedure were included. For adjustment of all-cause death during follow-up, age, sex, heart rate, systolic blood pressure, left ventricular ejection fraction (LVEF), history of DM, history of myocardial infarction, history of PCI, history of hypertension, current smoking, eGFR, DAPT, glycoprotein Ⅱb/Ⅲa inhibitor, angiotensin converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARB), parenteral anticoagulation therapy, coronary anatomy, implanted stents number, total implanted stents length, the diagnosis of NSTE-ACS subtypes, and the interval from admission to PCI procedure were included in multivariable analyses.
Subgroup analyses were conducted for major bleeding during follow-up. For each outcome, the most clinically relevant subgroups were analyzed. For each subgroup, the multivariable analysis was performed by adjusting all other subgroup factors. Forest plots were used to show the results.
All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC). All significant interactions were also examined. A two-sided P < 0.05 was considered statistically significant.
Of 6283 patients enrolled, 4705 (74.9%) were male, and 2143 (34.1%) had a history of T2DM, with a mean age of 64.13 ± 10.32 years. The median follow-up duration was 3.21 years. Among these patients, 3497 were diagnosed with NSTEMI (55.7%), and 2786 were diagnosed with unstable angina (44.3%). Based on baseline HbA1c levels, 4740 patients had the levels of admission HbA1c < 7%, and 1543 patients had the levels of admission HbA1c ≥ 7%. Patients with baseline HbA1c levels ≥ 7% were older and more likely to have higher heart rate, systolic blood pressure, serum creatinine levels, history of T2DM, and chronic heart failure than those with baseline HbA1c levels < 7%. Additionally, those with baseline HbA1c levels ≥ 7% had lower hematocrit and eGFR. Among patients with baseline HbA1c levels ≥ 7%, 1365 were diagnosed with T2DM, and 30.1% were administrated insulin. In patients with baseline HbA1c levels < 7%, 778 patients had T2DM, and 1.8% received insulin (Table 1).
| Characteristics | HbA1c ≥ 7% (n = 1543) | HbA1c < 7% (n = 4740) | Total (n = 6283) | P-value |
| Age | ||||
| Mean, yrs | 64.72 ± 9.73 | 63.93 ± 10.50 | 64.13 ± 10.32 | 0.007 |
| ≥ 65 yrs, | 806 (52.2%) | 2343 (49.4%) | 3149 (50.1%) | 0.056 |
| Female | 471 (30.5%) | 1107 (23.4%) | 1578 (25.1%) | < 0.001 |
| Weight, kg | 67.79 ± 12.21 | 65.89 ± 11.77 | 66.36 ± 11.90 | < 0.001 |
| Heart rate, beats/min | 75.72 ± 11.96 | 73.13 ± 11.33 | 73.77 ± 11.54 | < 0.001 |
| Blood pressure, mmHg | ||||
| Systolic | 136.70 ± 19.63 | 133.89 ± 19.59 | 134.58 ± 19.64 | < 0.001 |
| Diastolic | 77.58 ± 11.54 | 77.63 ± 11.74 | 77.62 ± 11.69 | 0.873 |
| LVEF, % | 60.39% ± 10.92% | 62.19% ± 9.65% | 61.75% ± 10.01% | < 0.001 |
| History of disease and risk factors | ||||
| Myocardial infarction | 263 (17.0%) | 710 (15.0%) | 973 (15.5%) | 0.051 |
| PCI | 332 (21.5%) | 758 (16.0%) | 1090 (17.3%) | < 0.001 |
| CABG | 20 (1.3%) | 49 (1.0%) | 69 (1.1%) | 0.390 |
| Stroke | 116 (7.5%) | 338 (7.1%) | 454 (7.2%) | 0.610 |
| Chronic heart failure | 237 (15.4%) | 578 (12.2%) | 815 (13.0%) | 0.001 |
| Atrial fibrillation | 55 (3.6%) | 157 (3.3%) | 212 (3.4%) | 0.634 |
| Current smoker | 372 (24.1%) | 1412 (29.8%) | 1784 (28.4%) | < 0.001 |
| Diabetes mellitus | 1365 (88.5%) | 778 (16.4%) | 2143 (34.1%) | < 0.001 |
| Hypertension | 1142 (74.0%) | 3107 (65.5%) | 4249 (67.6%) | < 0.001 |
| Hyperlipemia | 339 (22.0%) | 1062 (22.4%) | 1401 (22.3%) | 0.722 |
| eGFR | ||||
| Mean, mL/min per 1.73 m2 | 80.94 ± 28.33 | 83.19 ± 24.19 | 82.64 ± 25.29 | 0.005 |
| ≤ 60 mL/min per 1.73 m2 | 357 (23.1%) | 699 (14.7%) | 1056 (16.8%) | < 0.001 |
| Serum creatinine level, mg/dL | 1.07 ± 0.70 | 1.02 ± 0.63 | 1.03 ± 0.65 | 0.017 |
| Anemia | 531 (34.4%) | 1401 (29.6%) | 1932 (30.7%) | < 0.001 |
| Haemoglobin, g/L | 132.00 ± 17.51 | 134.42 ± 16.45 | 133.82 ± 16.75 | < 0.001 |
| Type of disease, | ||||
| NSTEMI | 677 (43.9%) | 2109 (44.5%) | 2786 (44.3%) | 0.671 |
| UA | 866 (56.1%) | 2631 (55.5%) | 3497 (55.7%) | |
| In-hospital medication | ||||
| Aspirin | 1503 (97.4%) | 4631 (97.7%) | 6134 (97.6%) | 0.431 |
| Clopidogrel | 1530 (99.2%) | 4699 (99.1%) | 6229 (99.1%) | 0.007 |
| Ticagrelor | 3 (0.2%) | 8 (0.2%) | 11 (0.2%) | 0.044 |
| Dual antiplatelet therapy | 1496 (97.0%) | 4604 (97.1%) | 6100 (97.1%) | 0.720 |
| Statin | 1511 (97.9%) | 4648 (98.1%) | 6159 (98.0%) | 0.744 |
| Warfarin | 13 (0.8%) | 20 (0.4%) | 33 (0.5%) | 0.047 |
| ACEI/ARB | 1270 (82.3%) | 3655 (77.1%) | 4925 (78.4%) | < 0.001 |
| Beta blockers | 1300 (84.3%) | 3961 (83.6%) | 5261 (83.7%) | 0.526 |
| Parenteral anticoagulation therapy | 592 (38.4%) | 1895 (40.0%) | 2487 (39.6%) | 0.261 |
| Glycoprotein IIb/IIIa inhibitor | 186 (12.1%) | 525 (11.1%) | 711 (11.3%) | 0.292 |
| Hypoglycemic therapy | ||||
| Insulin | 322 (30.1%) | 58 (1.8%) | 380 (9.0%) | < 0.001 |
| hypoglycemic drugs | 748 (70.0%) | 358 (11.3%) | 1106 (26.1%) | < 0.001 |
| Positive cardiac biomarkers | 820 (55.2%) | 2460 (53.7%) | 3280 (54.1%) | 0.315 |
| Procedure characteristics | ||||
| Access site | ||||
| Transradial access | 1312 (85.0%) | 4076 (86.0%) | 5388 (85.8%) | 0.347 |
| Transfemoral access | 231 (15.0%) | 664 (14.0%) | 895 (14.2%) | |
| Coronary anatomy | ||||
| Left main | 188 (12.2%) | 576 (12.2%) | 764 (12.2%) | |
| Multivessel disease | 1077 (69.8%) | 2928 (61.8%) | 4005 (63.7%) | < 0.001 |
| Other | 278 (18.0%) | 1236 (26.1%) | 1514 (24.1%) | |
| Stents number | 2.13 ± 1.22 | 1.99 ± 1.14 | 2.02 ± 1.16 | < 0.001 |
| Total length of stents, mm | 46 (28-72) | 42 (24-66) | 42 (24-68) | < 0.001 |
| Interval between admission and procedure | ||||
| Median, days | 2 (1-4) | 2 (1-3) | 2 (1-4) | 0.021 |
| < 24 h | 628 (40.7%) | 2064 (43.5%) | 2692 (42.8%) | |
| 24-72 h | 500 (32.4%) | 1497 (31.6%) | 1997 (31.8%) | 0.115 |
| > 72 h | 415 (26.9%) | 1179 (24.9%) | 1594 (25.4%) | |
| Data are presented as n (%) or median (IQR). ACEI: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers; BMS: bare metal stent; CABG: coronary artery bypass graft; DES: drug eluting stent; eGFR: estimated glomerular filtration rate; HbA1c: hemoglobin A1c; IQR: interquartile range; LVEF: left ventricular ejection fraction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; PTCA: percutaneous transluminal coronary angioplasty; UA: unstable angina. | ||||
As to in-hospital outcomes, there was no difference between patients with HbA1c < 7% and patients with HbA1c ≥ 7% in all-cause death, MACEs and any bleeding, but those whose HbA1c ≥ 7% had higher incidence of major bleeding. (1.5% vs. 2.3%, P = 0.038; Table 2). After adjusted by multivariable models, baseline HbA1c levels ≥7% did not show association with a higher risk of major bleeding (adjusted OR: 1.40, 95% CI: 0.81-2.44, P = 0.230), all-cause death (adjusted OR: 0.19, 95% CI: 0.02-1.49, P = 0.114), MACEs (adjusted OR: 0.87, 95% CI: 0.44-1.74, P = 0.703), and any bleeding (adjusted OR: 0.86, 95% CI: 0.68-1.09, P = 0.218; Table 2 and Table 4).
| Outcome | HbA1c ≥ 7% (n = 1543) | HbA1c < 7% (n = 4740) | Total (n = 6283) | P-value |
| All-cause death | 1 (0.1%) | 11 (0.2%) | 12 (0.2%) | 0.191 |
| MACEs | 11 (0.7%) | 35 (0.7%) | 46 (0.7%) | 0.919 |
| TVR | 0 | 4 (0.1%) | 4 (0.1%) | 0.254 |
| Ischemic stroke | 5 (0.3%) | 7 (0.1%) | 12 (0.2%) | 0.168 |
| Myocardial infarction | 5 (0.3%) | 18 (0.4%) | 23 (0.4%) | 0.753 |
| Major bleeding | 36 (2.3%) | 73 (1.5%) | 109 (1.7%) | 0.038 |
| Any bleeding | 191 (12.4%) | 627 (13.2%) | 818 (13.0%) | 0.389 |
| Data are presented as n (%). HbA1c: Hemoglobin A1c; MACEs: major adverse cardiovascular events; TVR: target vessel revascularization. | ||||
| Outcome | HbA1c ≥ 7% (n = 1543) | HbA1c < 7% (n = 4740) | Total (n = 6283) | P-value |
| 30 days | ||||
| All-cause death | 2 (0.1%) | 17 (0.4%) | 19 (0.3%) | 0.155 |
| Major bleeding | 37 (2.4%) | 73 (1.5%) | 110 (1.8%) | 0.026 |
| Any bleeding | 196 (12.7%) | 631 (13.3%) | 827 (13.2%) | 0.538 |
| 1 year | ||||
| All-cause death | 29 (1.9%) | 89 (1.9%) | 118 (1.9%) | 0.996 |
| Major bleeding | 43 (2.8%) | 81 (1.7%) | 124 (2.0%) | 0.008 |
| Any bleeding | 230 (14.9%) | 719 (15.2%) | 949 (15.1%) | 0.802 |
| 3 years | ||||
| All-cause death | 91 (5.9%) | 216 (4.6%) | 307 (4.9%) | 0.034 |
| Major bleeding | 55 (3.6%) | 106 (2.2%) | 161 (2.6%) | 0.004 |
| Any bleeding | 280 (18.1%) | 887 (18.7%) | 1167 (18.6%) | 0.619 |
| 5 years | ||||
| All-cause death | 129 (8.4%) | 297 (6.3%) | 426 (6.8%) | 0.004 |
| Major bleeding | 57 (3.7%) | 110 (2.3%) | 167 (2.7%) | 0.004 |
| Any bleeding | 290 (18.8%) | 911 (19.2%) | 1201 (19.1%) | 0.712 |
| HbA1c: Hemoglobin A1c. | ||||
| Outcomes | Univariate analysis | Multivariate analysis | |||||
| Odds or hazard ratio | 95% CI | P-value | Odds or hazard ratio | 95% CI | P value | ||
| In-hospital | |||||||
| All-cause death | 0.28 | 0.04-2.16 | 0.222 | 0.19 | 0.02-1.49 | 0.114 | |
| MACEs | 0.97 | 0.49-1.91 | 0.919 | 0.87 | 0.44-1.74 | 0.703 | |
| Major bleeding | 1.53 | 1.02-2.29 | 0.040 | 1.40 | 0.81-2.44 | 0.230 | |
| Any bleeding | 0.93 | 0.78-1.10 | 0.389 | 0.86 | 0.68-1.09 | 0.218 | |
| Follow up | |||||||
| All-cause death | 1.34 | 1.09-1.64 | 0.006 | 0.88 | 0.66-1.18 | 0.398 | |
| Major bleeding | 1.61 | 1.17-2.22 | 0.004 | 1.57 | 1.01-2.44 | 0.044 | |
| Any bleeding | 0.98 | 0.86-1.12 | 0.772 | 1.00 | 0.84-1.19 | 0.994 | |
| MACEs: major adverse cardiovascular events. | |||||||
Compared with the patients with HbA1c < 7%, the risk of major bleeding events was higher in the patients with HbA1c ≥ 7% during long-term follow-up but not in short-term follow-up (in 30 days: adjusted HR = 1.48; 95% CI: 0.86-2.55; P = 0.153, in the whole follow-up: adjusted HR = 1.57; 95% CI: 1.01-2.44; P = 0.044; Table 3 and Table 4). No difference was observed in all-cause death and any bleeding between the patients with HbA1c < 7% and with HbA1c ≥ 7% (all-cause death: adjusted HR = 0.88; 95% CI: 0.66-1.18; P = 0.398; any bleeding: adjusted HR = 1.00, 95% CI: 0.84-1.19, P = 0.994; Table 3 and Table 4). After adjustment of potential confounders included in multivariable analyses, Cox regression plots showed that the patients with HbA1c ≥ 7% had more major bleeding events (P = 0.044), whereas the all-cause death occurred similarly in both patients with HbA1c ≥ 7% and with HbA1c < 7% (P = 0.398; Figure S1 in supplemental materials). As to the Kaplan-Meier analyses for major bleeding, more major bleeding occurred in patients with HbA1c ≥ 7% in the first 30 days, from 30 days to 6 years and the whole duration of follow-up (P = 0.027, P = 0.049, P = 0.003, respectively; Figure 2). The long-term mortality was lower in those patients with HbA1c < 7% (P = 0.005; Figure 2).
Generally, the association between baseline glycemic status on admission and major bleeding was consistent across subgroups with primary analyses. There was no significant interaction between baseline HbA1c levels and other baseline characteristics factors, including the previous diagnosis of T2DM and chronic kidney disease (P for interaction > 0.10 in all comparisons; Figure 3). Also, no significant interaction between baseline HbA1c levels and insulin use or other hypoglycemic treatment use (P for interaction = 0.177 and = 0.674, respectively; Figure S2 in supplemental materials).
This study aimed to investigate the association between baseline HbA1c levels and bleeding in NSTE-ACS patients undergoing PCI. We found that compared with baseline HbA1c < 7%, baseline HbA1c levels ≥ 7% were associated with a higher long-term risk of major bleeding but not associated with all-cause death in patients with NSTE-ACS undergoing PCI.
Compared with NSTE-ACS patients who received conservative strategies, the patients undergoing PCI who were administered additional anticoagulants during the procedure had a higher risk of bleeding events.[11,12] Although DM was reported as a risk factor both for ischemic events and bleeding in NSTEMI patients,[5] limited studies have investigated the association between DM and bleeding events in NSTE-ACS patients undergoing PCI.
There are inconsistent observations in the association between HbA1c levels with all-cause death. The previous studies showed that baseline HbA1c was an independent predictive factor for all-cause death regardless of whether the patients had a previous diagnosis of DM.[10,15,16] Recently, Baber, et al.[9] reported that in patients with ACS undergoing PCI, baseline HbA1c levels display a U-shaped association with long-term all-cause death or myocardial infarction, where the lower HbA1c levels were also related to a higher ischemic event. Engoren, et al.[17] also reported that patients with lower HbA1c undergoing CABG had more cardiovascular death events.
In the present study, the crude K-M curve showed that relative to baseline HbA1c < 7%, baseline HbA1c levels ≥ 7% were associated with more all-cause death events. After adjustment with several potential confounders, there was no significance in the association between HbA1c levels and all-cause death, which possibly suggested that several confounders raised from higher HbA1c levels may be adjusted in this study.
Diabetes was identified as associated with major bleeding events, including gastrointestinal bleeding and intracranial bleeding.[18] The Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines (CRUSADE) bleeding score enrolled the history of diabetes as an independent predictive factor for post-MI bleeding risk.[5]
The current evidence showed inconsistent results. Consistent with the CRUSADE study, Giraldez, et al.[15] found that in patients with NSTE-ACS, compared with those who had normal glycemic status, those who had DM experienced a higher risk of bleeding events defined by GUSTO moderate or severe bleeding. On the contrary, several recent studies failed to confirm the roles of DM in increasing the risk of bleeding in patients with coronary artery diseases undergoing PCI.[19-22] Given that HbA1c levels were considered a better reflection of the glycemic status of patients than instant blood glucose or the history of DM, it might play a more crucial role in maintaining the balance between ischemia and bleeding.[23] In the latest study, Samuelsen, et al.[24] reported DM defined by HbA1c was identified as the risk factor for the higher risk of major bleeding in patients undergoing PCI. Additionally, a previous study conducted on patients with stable coronary diseases reported that the higher baseline HbA1c was also associated with a higher incidence of major bleeding (BARC > 2 grade) in patients.[25] The subgroup analysis of the PLATO study also showed that the higher HbA1c was associated with a higher incidence of bleeding events in ACS patients receiving invasive or non-invasive strategies. However, there was limited evidence to investigate the association of HbA1c with bleeding events in NSTE-ACS patients undergoing PCI.
In this study, we found that the NSTE-ACS patients with higher baseline HbA1c levels undergoing PCI had more incidences of major bleeding (3-5 grade). The reasons why high HbA1c levels are associated with bleeding remain unclear. Lemesle et al. thought physicians would tend to provide more stringent secondary prevention to DM patients for reducing the incidence of ischemic events. Still, these strategies may increase the risk of bleeding in turn.[25] Additionally, the patients who were enrolled in the CORONOR study and EARLY ACS study where the higher HbA1c levels or DM were associated with higher bleeding risk, might be more administrated aspirin and clopidogrel.[15,25] The previous study suggested that clopidogrel usage may increase the risk of bleeding in patients with DM.[22,24] There also were a high proportion of aspirin use and clopidogrel use in our study (aspirin: 100.0% and clopidogrel: 99.1%), which may enhance the potential effect of HbA1c on bleeding in patients with NSTE-ACS undergoing PCI, but the clear mechanism should be further elucidated.
Moreover, we noticed that new major bleeding events burst in the first 30 days and the accumulation trend flatten from 30 days to 6-year follow-up. This trend may suggest the impact of HbA1c levels on bleeding events throughout in-hospital antithrombotic medication and discharge antiplatelet management in general. Also, the glucose-lowering drugs, including metformin and sulfonylureas, may interact with antithrombotic therapies to decrease platelet activity, which may be the underlying reason for a higher incidence of bleeding events in this study.[26] Another potential mechanism could be attributed to the higher PAI-1 and t-PA levels indicating a disorder in the fibrinolytic system in DM patients.[27] Hyperglycemia status and increased insulin levels can stimulate the production of PAI-1 by endothelial cells and adipocytes.[28-31] The increase in PAI-1 reduces the serine protease effect of rtPA, which may produce increased endothelial damage, blood-brain barrier breakdown, and microvasculature, leading to hemorrhagic complications.[32,33] However, clearer mechanisms of the relation between glycemic status and bleeding still should be further studied.
Although the previous studies found that the administration of insulin increased the incidence of mortality,[34] this was not associated with the higher risk of bleeding events in patients with ACS.[19] Consistently, in the present study, the subgroup analyses failed to find the differences in the association between the risk of bleeding and HbA1c levels in the patients with or without insulin or hypoglycemic treatments. These indicated that further studies are required for investigating the association between insulin or hypoglycemic treatments and bleeding in patients with NSTE-ACS undergoing PCI.
First, this study is an observational cohort study, meaning several potential confounders impacted the results. We minimized this impact by enrolling multivariate analyses, but there still are residual confounders unadjusted. Second, the low incidence of events makes subgroup analyses insufficient to find the predictive effect of baseline HbA1c levels in patients with DM or without DM. Alternatively, further studies could enroll the NSTE-ACS patients with or without DM respectively to demonstrate the roles of baseline HbA1c in the prediction of bleeding events in a different group. Third, this study didn’t record the HbA1c levels during follow-up or long-term glucose-lowering strategies. Therefore, it could not determine the long-term glycemic status on clinical outcomes in patients undergoing PCI for NSTE-ACS.
Compared with the lower baseline HbA1c levels, the higher baseline HbA1c levels were associated with an increase in long-term bleeding risk in NSTE-ACS patients undergoing PCI, though higher baseline HbA1c levels were not associated with a higher risk of mortality.
The study protocol was approved by the central ethics committee of the Guangdong Provincial People’s Hospital, Guangzhou, China, with a waiver of informed consent (No. GDREC2016210H(R1)).
This work was supported by Outstanding Young Medical Talents in Guangdong Province (KJ012019456), the Outstanding Young Talent Program of Guangdong Provincial People’s Hospital (Grant No. KJ012019084 and KJ012019095), the High-level Hospital Construction Project (Grant No. DFJH2020021), Guangdong Provincial People’s Hospital Clinical Research Fund (Y012018085), and National Natural Science Foundation of Guangdong Province (KC022021005).
None.
| [1] |
Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 267−315. doi: 10.1093/eurheartj/ehv320
|
| [2] |
Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA 2007; 298: 765−775. doi: 10.1001/jama.298.7.765
|
| [3] |
Dotevall A, Hasdai D, Wallentin L, et al. Diabetes mellitus: clinical presentation and outcome in men and women with acute coronary syndromes. Data from the Euro Heart Survey ACS. Diabet Med 2005; 22: 1542−1550.
|
| [4] |
Godoy LC, Lawler PR, Farkouh ME, et al. Urgent revascularization strategies in patients with diabetes mellitus and acute coronary syndrome. Can J Cardiol 2019; 35: 993−1001. doi: 10.1016/j.cjca.2019.03.010
|
| [5] |
Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009; 119: 1873−1882. doi: 10.1161/CIRCULATIONAHA.108.828541
|
| [6] |
Liu R, Lyu SZ, Zhao GQ, et al. Comparison of the performance of the CRUSADE, ACUITY-HORIZONS, and ACTION bleeding scores in ACS patients undergoing PCI: insights from a cohort of 4939 patients in China. J Geriatr Cardiol 2017; 14: 93−99.
|
| [7] |
Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020; 41: 255−323. doi: 10.1093/eurheartj/ehz486
|
| [8] |
Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019; 40: 87−165. doi: 10.1093/eurheartj/ehy394
|
| [9] |
Baber U, Azzalini L, Masoomi R, et al. Hemoglobin A1c and cardiovascular outcomes following percutaneous coronary intervention: insights from a large single-center registry. JACC Cardiovasc Interv 2021; 14: 388−397. doi: 10.1016/j.jcin.2020.10.008
|
| [10] |
James S, Angiolillo DJ, Cornel JH, et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes:a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J 2010; 31: 3006−3016.
|
| [11] |
Lopes RD, Subherwal S, Holmes DN, et al. The association of in-hospital major bleeding with short-, intermediate-, and long-term mortality among older patients with non-ST-segment elevation myocardial infarction. Eur Heart J 2012; 33: 2044−2053. doi: 10.1093/eurheartj/ehs012
|
| [12] |
Hoenig MR, Doust JA, Aroney CN, Scott IA. Early invasive versus conservative strategies for unstable angina & non-ST-elevation myocardial infarction in the stent era. Cochrane Database Syst Rev 2006: CD004815.
|
| [13] |
Chen JY, He PC, Liu YH, et al. Association of parenteral anticoagulation therapy with outcomes in chinese patients undergoing percutaneous coronary intervention for non–ST-segment elevation acute coronary syndrome. JAMA Internal Medicine 2019; 179: 186. doi: 10.1001/jamainternmed.2018.5953
|
| [14] |
Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123: 2736−747. doi: 10.1161/CIRCULATIONAHA.110.009449
|
| [15] |
Giraldez RR, Clare RM, Lopes RD, et al. Prevalence and clinical outcomes of undiagnosed diabetes mellitus and prediabetes among patients with high-risk non-ST-segment elevation acute coronary syndrome. Am Heart J 2013; 165: 918−25 e2.
|
| [16] |
Timmer JR, Hoekstra M, Nijsten MW, et al. Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation 2011; 124: 704−711. doi: 10.1161/CIRCULATIONAHA.110.985911
|
| [17] |
Engoren M, Schwann TA, Arslanian-Engoren C, et al. U-shape association between hemoglobin A1c and late mortality in patients with heart failure after cardiac surgery. Am J Cardiol 2013; 111: 1209−1213. doi: 10.1016/j.amjcard.2012.12.054
|
| [18] |
De Berardis G, Lucisano G, D'Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA 2012; 307: 2286−2294.
|
| [19] |
Chichareon P, Modolo R, Kogame N, et al. Association of diabetes with outcomes in patients undergoing contemporary percutaneous coronary intervention: Pre-specified subgroup analysis from the randomized GLOBAL LEADERS study. Atherosclerosis 2020; 295: 45−53. doi: 10.1016/j.atherosclerosis.2020.01.002
|
| [20] |
Ploumen EH, Pinxterhuis TH, Zocca P, et al. Impact of prediabetes and diabetes on 3-year outcome of patients treated with new-generation drug-eluting stents in two large-scale randomized clinical trials. Cardiovasc Diabetol 2021; 20: 217. doi: 10.1186/s12933-021-01405-4
|
| [21] |
Cavallari I, Maddaloni E, Gragnano F, et al. Ischemic and bleeding risk by type 2 diabetes clusters in patients with acute coronary syndrome. Intern Emerg Med 2021; 16: 1583−1591. doi: 10.1007/s11739-021-02640-z
|
| [22] |
Simek S, Motovska Z, Hlinomaz O, et al. The effect of diabetes on prognosis following myocardial infarction treated with primary angioplasty and potent antiplatelet therapy. J Clin Med 2020; 9: 2555. doi: 10.3390/jcm9082555
|
| [23] |
Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010; 362: 800−811. doi: 10.1056/NEJMoa0908359
|
| [24] |
Samuelsen PJ, Eggen AE, Steigen T, et al. Incidence and risk factors for major bleeding among patients undergoing percutaneous coronary intervention: Findings from the Norwegian Coronary Stent Trial (NORSTENT). PLoS One 2021; 16: e0247358. doi: 10.1371/journal.pone.0247358
|
| [25] |
Lemesle G, Meurice T, Tricot O, et al. Association of diabetic status and glycemic control with ischemic and bleeding outcomes in patients with stable coronary artery disease: The 5-Year CORONOR Registry. J Am Heart Assoc 2018; 7: e008354. doi: 10.1161/JAHA.117.008354
|
| [26] |
Nusca A, Tuccinardi D, Pieralice S, et al. Platelet effects of anti-diabetic therapies: new perspectives in the management of patients with diabetes and cardiovascular disease. Front Pharmacol 2021; 12: 670155. doi: 10.3389/fphar.2021.670155
|
| [27] |
Meigs JB, Mittleman MA, Nathan DM, et al. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the Framingham Offspring Study. JAMA 2000; 283: 221−228. doi: 10.1001/jama.283.2.221
|
| [28] |
Pandolfi A, Iacoviello L, Capani F, et al. Glucose and insulin independently reduce the fibrinolytic potential of human vascular smooth muscle cells in culture. Diabetologia 1996; 39: 1425−1431. doi: 10.1007/s001250050594
|
| [29] |
Morange PE, Aubert J, Peiretti F, et al. Glucocorticoids and insulin promote plasminogen activator inhibitor 1 production by human adipose tissue. Diabetes 1999; 48: 890−895. doi: 10.2337/diabetes.48.4.890
|
| [30] |
Alessi MC, Peiretti F, Morange P, et al. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes 1997; 46: 860−867. doi: 10.2337/diab.46.5.860
|
| [31] |
Nordt TK, Klassen KJ, Schneider DJ, Sobel BE. Augmentation of synthesis of plasminogen activator inhibitor type-1 in arterial endothelial cells by glucose and its implications for local fibrinolysis. Arterioscler Thromb 1993; 13: 1822−1828. doi: 10.1161/01.ATV.13.12.1822
|
| [32] |
Shatos MA, Doherty JM, Penar PL, Sobel BE. Suppression of plasminogen activator inhibitor-1 release from human cerebral endothelium by plasminogen activators. A factor potentially predisposing to intracranial bleeding. Circulation 1996; 94: 636−642.
|
| [33] |
Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol 2010; 6: 145−155. doi: 10.1038/nrneurol.2009.231
|
| [34] |
Sharma PK, Agarwal S, Ellis SG, et al. Association of glycemic control with mortality in patients with diabetes mellitus undergoing percutaneous coronary intervention. Circ Cardiovasc Interv 2014; 7: 503−509. doi: 10.1161/CIRCINTERVENTIONS.113.001107
|
| [1] | Jin-Wen TIAN, Mei ZHU, Feng-Qi WANG, Ke LI, Chao-Fei ZHOU, Bo LI, Min WANG, Jue-Lin DENG, Bo JIANG, Jing BAI, Yi GUO, Rong-Jie JIN, Zhao ZHANG, Ying LIN, Ji-Hang WANG, Shi-Hao ZHAO, Ming-Zhi SHEN. Intracoronary arterial retrograde thrombolysis with percutaneous coronary intervention: a novel use of thrombolytic to treat acute ST-segment elevation myocardial infarction[J]. Journal of Geriatric Cardiology, 2019, 16(6): 458-467. DOI: 10.11909/j.issn.1671-5411.2019.06.004 |
| [2] | Xiao-Qing CAI, Feng TIAN, Shan-Shan ZHOU, Jing JING, Wei HU, Tao ZHANG, Xi WANG, Ri-Na DU, Qiang XU, Yun-Dai CHEN. A rare case of non-ST-segment elevation myocardial infarction triggered by coronary subclavian steal syndrome[J]. Journal of Geriatric Cardiology, 2019, 16(4): 378-380. DOI: 10.11909/j.issn.1671-5411.2019.04.007 |
| [3] | Miquel Vives-Borrás, Manuel Martínez-Sellés, Albert Ariza-Solé, María T. Vidán, Francesc Formiga, Héctor Bueno, Juan Sanchís, Oriol Alegre, Albert Durán-Cambra, Ramón López-Palop, Emad Abu-Assi, Alessandro Sionis, LONGEVO-SCA Investigators. Clinical and prognostic implications of delirium in elderly patients with non–ST-segment elevation acute coronary syndromes[J]. Journal of Geriatric Cardiology, 2019, 16(2): 121-128. DOI: 10.11909/j.issn.1671-5411.2019.02.008 |
| [4] | Xue–Dong ZHAO, Guan–Qi ZHAO, Xiao WANG, Shu–Tian SHI, Wen ZHENG, Rui–Feng GUO, Shao–Ping NIE. Optimal timing of staged percutaneous coronary intervention in ST-segment elevation myocardial infarction patients with multivessel disease[J]. Journal of Geriatric Cardiology, 2018, 15(5): 356-362. DOI: 10.11909/j.issn.1671-5411.2018.05.005 |
| [5] | Ran LIU, Shu-Zheng LYU, Guan-Qi ZHAO, Wen ZHENG, Xiao WANG, Xue-Dong ZHAO, Sheng-Hui ZHOU, Lei ZHEN, Shao-Ping NIE. Comparison of the performance of the CRUSADE, ACUITY-HORIZONS, and ACTION bleeding scores in ACS patients undergoing PCI: insights from a cohort of 4939 patients in China[J]. Journal of Geriatric Cardiology, 2017, 14(2): 93-99. DOI: 10.11909/j.issn.1671-5411.2017.02.011 |
| [6] | Xiao-Fan YU, Yi LI, Qian-Cheng WANG, Xiao-Zeng WANG, Ming LIANG, Xin ZHAO, Kai XU, Ya-Ling HAN. Staged versus “one-time” multivessel intervention in elderly patients with non-ST-elevation acute coronary syndrome[J]. Journal of Geriatric Cardiology, 2016, 13(9): 760-767. DOI: 10.11909/j.issn.1671-5411.2016.09.004 |
| [7] | Tian-Wen HAN, Shan-Shan ZHOU, Jian-Tao LI, Feng TIAN, Yang MU, Jing JING, Yun-Feng HAN, Yun-Dai CHEN. Homocysteine is associated with the progression of non-culprit coronary lesions in elderly acute coronary syndrome patients after percutaneous coronary intervention[J]. Journal of Geriatric Cardiology, 2016, 13(4): 299-305. DOI: 10.11909/j.issn.1671-5411.2016.04.010 |
| [8] | Chun-Peng MA, Xiao WANG, Qing-Sheng WANG, Xiao-Li LIU, Xiao-Nan HE, Shao-Ping NIE. A modified HEART risk score in chest pain patients with suspected non-ST-segment elevation acute coronary syndrome[J]. Journal of Geriatric Cardiology, 2016, 13(1): 64-69. DOI: 10.11909/j.issn.1671-5411.2016.01.013 |
| [9] | Li-Xiang MA, Zhen-Hua LU, Le WANG, Xin DU, Chang-Sheng MA. Culprit vessel only versus “one-week” staged percutaneous coronary intervention for multivessel disease in patients presenting with ST-segment elevation myocardial infarction[J]. Journal of Geriatric Cardiology, 2015, 12(3): 226-231. DOI: 10.11909/j.issn.1671-5411.2015.03.001 |
| [10] | Kwang Sun Ryu, Hyun Woo Park, Soo Ho Park, Ho Sun Shon, Keun Ho Ryu, Dong Gyu Lee, Mohamed EA Bashir, Ju Hee Lee, Sang Min Kim, Sang Yeub Lee, Jang Whan Bae, Kyung Kuk Hwang, Dong Woon Kim, Myeong Chan Cho, Young Keun Ahn, Myung Ho Jeong, Chong Jin Kim, Jong Seon Park, Young Jo Kim, Yang Soo Jang, Hyo Soo Kim, Ki Bae Seung, Other Korea Acute Myocardial Infarction Registry Investigators. Comparison of clinical outcomes between culprit vessel only and multivessel percutaneous coronary intervention for ST-segment elevation myocardial infarction patients with multivessel coronary diseases[J]. Journal of Geriatric Cardiology, 2015, 12(3): 208-217. DOI: 10.11909/j.issn.1671-5411.2015.03.014 |
| 1. | Wang M, Su W, Chen H, et al. The joint association of diabetes status and NT-ProBNP with adverse cardiac outcomes in patients with non-ST-segment elevation acute coronary syndrome: a prospective cohort study. Cardiovasc Diabetol, 2023, 22(1): 46. DOI:10.1186/s12933-023-01771-1 |
| Characteristics | HbA1c ≥ 7% (n = 1543) | HbA1c < 7% (n = 4740) | Total (n = 6283) | P-value |
| Age | ||||
| Mean, yrs | 64.72 ± 9.73 | 63.93 ± 10.50 | 64.13 ± 10.32 | 0.007 |
| ≥ 65 yrs, | 806 (52.2%) | 2343 (49.4%) | 3149 (50.1%) | 0.056 |
| Female | 471 (30.5%) | 1107 (23.4%) | 1578 (25.1%) | < 0.001 |
| Weight, kg | 67.79 ± 12.21 | 65.89 ± 11.77 | 66.36 ± 11.90 | < 0.001 |
| Heart rate, beats/min | 75.72 ± 11.96 | 73.13 ± 11.33 | 73.77 ± 11.54 | < 0.001 |
| Blood pressure, mmHg | ||||
| Systolic | 136.70 ± 19.63 | 133.89 ± 19.59 | 134.58 ± 19.64 | < 0.001 |
| Diastolic | 77.58 ± 11.54 | 77.63 ± 11.74 | 77.62 ± 11.69 | 0.873 |
| LVEF, % | 60.39% ± 10.92% | 62.19% ± 9.65% | 61.75% ± 10.01% | < 0.001 |
| History of disease and risk factors | ||||
| Myocardial infarction | 263 (17.0%) | 710 (15.0%) | 973 (15.5%) | 0.051 |
| PCI | 332 (21.5%) | 758 (16.0%) | 1090 (17.3%) | < 0.001 |
| CABG | 20 (1.3%) | 49 (1.0%) | 69 (1.1%) | 0.390 |
| Stroke | 116 (7.5%) | 338 (7.1%) | 454 (7.2%) | 0.610 |
| Chronic heart failure | 237 (15.4%) | 578 (12.2%) | 815 (13.0%) | 0.001 |
| Atrial fibrillation | 55 (3.6%) | 157 (3.3%) | 212 (3.4%) | 0.634 |
| Current smoker | 372 (24.1%) | 1412 (29.8%) | 1784 (28.4%) | < 0.001 |
| Diabetes mellitus | 1365 (88.5%) | 778 (16.4%) | 2143 (34.1%) | < 0.001 |
| Hypertension | 1142 (74.0%) | 3107 (65.5%) | 4249 (67.6%) | < 0.001 |
| Hyperlipemia | 339 (22.0%) | 1062 (22.4%) | 1401 (22.3%) | 0.722 |
| eGFR | ||||
| Mean, mL/min per 1.73 m2 | 80.94 ± 28.33 | 83.19 ± 24.19 | 82.64 ± 25.29 | 0.005 |
| ≤ 60 mL/min per 1.73 m2 | 357 (23.1%) | 699 (14.7%) | 1056 (16.8%) | < 0.001 |
| Serum creatinine level, mg/dL | 1.07 ± 0.70 | 1.02 ± 0.63 | 1.03 ± 0.65 | 0.017 |
| Anemia | 531 (34.4%) | 1401 (29.6%) | 1932 (30.7%) | < 0.001 |
| Haemoglobin, g/L | 132.00 ± 17.51 | 134.42 ± 16.45 | 133.82 ± 16.75 | < 0.001 |
| Type of disease, | ||||
| NSTEMI | 677 (43.9%) | 2109 (44.5%) | 2786 (44.3%) | 0.671 |
| UA | 866 (56.1%) | 2631 (55.5%) | 3497 (55.7%) | |
| In-hospital medication | ||||
| Aspirin | 1503 (97.4%) | 4631 (97.7%) | 6134 (97.6%) | 0.431 |
| Clopidogrel | 1530 (99.2%) | 4699 (99.1%) | 6229 (99.1%) | 0.007 |
| Ticagrelor | 3 (0.2%) | 8 (0.2%) | 11 (0.2%) | 0.044 |
| Dual antiplatelet therapy | 1496 (97.0%) | 4604 (97.1%) | 6100 (97.1%) | 0.720 |
| Statin | 1511 (97.9%) | 4648 (98.1%) | 6159 (98.0%) | 0.744 |
| Warfarin | 13 (0.8%) | 20 (0.4%) | 33 (0.5%) | 0.047 |
| ACEI/ARB | 1270 (82.3%) | 3655 (77.1%) | 4925 (78.4%) | < 0.001 |
| Beta blockers | 1300 (84.3%) | 3961 (83.6%) | 5261 (83.7%) | 0.526 |
| Parenteral anticoagulation therapy | 592 (38.4%) | 1895 (40.0%) | 2487 (39.6%) | 0.261 |
| Glycoprotein IIb/IIIa inhibitor | 186 (12.1%) | 525 (11.1%) | 711 (11.3%) | 0.292 |
| Hypoglycemic therapy | ||||
| Insulin | 322 (30.1%) | 58 (1.8%) | 380 (9.0%) | < 0.001 |
| hypoglycemic drugs | 748 (70.0%) | 358 (11.3%) | 1106 (26.1%) | < 0.001 |
| Positive cardiac biomarkers | 820 (55.2%) | 2460 (53.7%) | 3280 (54.1%) | 0.315 |
| Procedure characteristics | ||||
| Access site | ||||
| Transradial access | 1312 (85.0%) | 4076 (86.0%) | 5388 (85.8%) | 0.347 |
| Transfemoral access | 231 (15.0%) | 664 (14.0%) | 895 (14.2%) | |
| Coronary anatomy | ||||
| Left main | 188 (12.2%) | 576 (12.2%) | 764 (12.2%) | |
| Multivessel disease | 1077 (69.8%) | 2928 (61.8%) | 4005 (63.7%) | < 0.001 |
| Other | 278 (18.0%) | 1236 (26.1%) | 1514 (24.1%) | |
| Stents number | 2.13 ± 1.22 | 1.99 ± 1.14 | 2.02 ± 1.16 | < 0.001 |
| Total length of stents, mm | 46 (28-72) | 42 (24-66) | 42 (24-68) | < 0.001 |
| Interval between admission and procedure | ||||
| Median, days | 2 (1-4) | 2 (1-3) | 2 (1-4) | 0.021 |
| < 24 h | 628 (40.7%) | 2064 (43.5%) | 2692 (42.8%) | |
| 24-72 h | 500 (32.4%) | 1497 (31.6%) | 1997 (31.8%) | 0.115 |
| > 72 h | 415 (26.9%) | 1179 (24.9%) | 1594 (25.4%) | |
| Data are presented as n (%) or median (IQR). ACEI: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers; BMS: bare metal stent; CABG: coronary artery bypass graft; DES: drug eluting stent; eGFR: estimated glomerular filtration rate; HbA1c: hemoglobin A1c; IQR: interquartile range; LVEF: left ventricular ejection fraction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; PTCA: percutaneous transluminal coronary angioplasty; UA: unstable angina. | ||||
| Outcome | HbA1c ≥ 7% (n = 1543) | HbA1c < 7% (n = 4740) | Total (n = 6283) | P-value |
| All-cause death | 1 (0.1%) | 11 (0.2%) | 12 (0.2%) | 0.191 |
| MACEs | 11 (0.7%) | 35 (0.7%) | 46 (0.7%) | 0.919 |
| TVR | 0 | 4 (0.1%) | 4 (0.1%) | 0.254 |
| Ischemic stroke | 5 (0.3%) | 7 (0.1%) | 12 (0.2%) | 0.168 |
| Myocardial infarction | 5 (0.3%) | 18 (0.4%) | 23 (0.4%) | 0.753 |
| Major bleeding | 36 (2.3%) | 73 (1.5%) | 109 (1.7%) | 0.038 |
| Any bleeding | 191 (12.4%) | 627 (13.2%) | 818 (13.0%) | 0.389 |
| Data are presented as n (%). HbA1c: Hemoglobin A1c; MACEs: major adverse cardiovascular events; TVR: target vessel revascularization. | ||||
| Outcome | HbA1c ≥ 7% (n = 1543) | HbA1c < 7% (n = 4740) | Total (n = 6283) | P-value |
| 30 days | ||||
| All-cause death | 2 (0.1%) | 17 (0.4%) | 19 (0.3%) | 0.155 |
| Major bleeding | 37 (2.4%) | 73 (1.5%) | 110 (1.8%) | 0.026 |
| Any bleeding | 196 (12.7%) | 631 (13.3%) | 827 (13.2%) | 0.538 |
| 1 year | ||||
| All-cause death | 29 (1.9%) | 89 (1.9%) | 118 (1.9%) | 0.996 |
| Major bleeding | 43 (2.8%) | 81 (1.7%) | 124 (2.0%) | 0.008 |
| Any bleeding | 230 (14.9%) | 719 (15.2%) | 949 (15.1%) | 0.802 |
| 3 years | ||||
| All-cause death | 91 (5.9%) | 216 (4.6%) | 307 (4.9%) | 0.034 |
| Major bleeding | 55 (3.6%) | 106 (2.2%) | 161 (2.6%) | 0.004 |
| Any bleeding | 280 (18.1%) | 887 (18.7%) | 1167 (18.6%) | 0.619 |
| 5 years | ||||
| All-cause death | 129 (8.4%) | 297 (6.3%) | 426 (6.8%) | 0.004 |
| Major bleeding | 57 (3.7%) | 110 (2.3%) | 167 (2.7%) | 0.004 |
| Any bleeding | 290 (18.8%) | 911 (19.2%) | 1201 (19.1%) | 0.712 |
| HbA1c: Hemoglobin A1c. | ||||
| Outcomes | Univariate analysis | Multivariate analysis | |||||
| Odds or hazard ratio | 95% CI | P-value | Odds or hazard ratio | 95% CI | P value | ||
| In-hospital | |||||||
| All-cause death | 0.28 | 0.04-2.16 | 0.222 | 0.19 | 0.02-1.49 | 0.114 | |
| MACEs | 0.97 | 0.49-1.91 | 0.919 | 0.87 | 0.44-1.74 | 0.703 | |
| Major bleeding | 1.53 | 1.02-2.29 | 0.040 | 1.40 | 0.81-2.44 | 0.230 | |
| Any bleeding | 0.93 | 0.78-1.10 | 0.389 | 0.86 | 0.68-1.09 | 0.218 | |
| Follow up | |||||||
| All-cause death | 1.34 | 1.09-1.64 | 0.006 | 0.88 | 0.66-1.18 | 0.398 | |
| Major bleeding | 1.61 | 1.17-2.22 | 0.004 | 1.57 | 1.01-2.44 | 0.044 | |
| Any bleeding | 0.98 | 0.86-1.12 | 0.772 | 1.00 | 0.84-1.19 | 0.994 | |
| MACEs: major adverse cardiovascular events. | |||||||